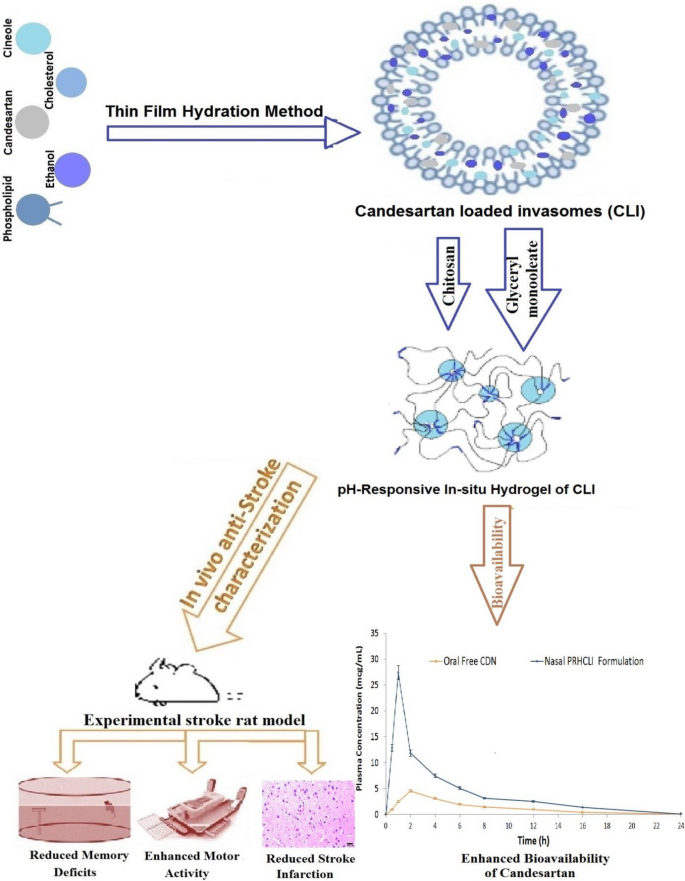
Candesartan
(CDN) is a useful anti-stroke medication because it lowers blood
pressure, inflammation, oxidative stress, angiogenesis and apoptosis.
However, CDN has limited efficacy due to its low solubility and poor
bioavailability. This study set out to develop nasal pH-responsive in
situ hydrogel of CDN-loaded invasomes a (PRHCLI) for enhancing CDN’s
release, penetration, bioavailability, and effectiveness as a possible
treatment for stroke. Based on the results of the pre-formulation
investigation, the optimum CLI formulation for intravasomal delivery of
CDN was determined to be 3% of phospholipid, 0.16% of cholesterol, 3% of
ethanol, and 1% of cineole. The optimum formulation significantly
enhanced CDN permeation and release by 2.06-fold and 59.06%,
respectively. The CLI formulation was added to a mixture of chitosan
(0.67%w/v) and glyceryl monooleate (0.27%v/v) to develop PRHCLI. The
PRHCLI formulation enhanced the release and permeation of CDN relative
to free CDN by 2.15 and 2.76 folds, respectively. An experimental rat
stroke model was utilized for in vivo studies to evaluate the
bioavailability, effectiveness, and toxicity of the PRHCLI formulation.
The nasal PRHCLI drops increased the CDN’s bioavailability by 3.20-fold
compared to oral free CDN. Increased grip strength and decreased
flexion, spontaneous motor activity, and Morris Water Maze scores in
comparison to oral free CDN showed that nasal PRHCLI drops have better
anti-stroke activity. The toxicity evaluation revealed the safety of
nasal PRHCLI. Hence, nasal PRHCLI drops may represent a promising avenue
as a stroke therapy.
Graphical abstract
No comments:
Post a Comment