This is where stroke leaders would look at this
and mutter to themselves; 'We could use this to detect brain signals
occurring during stroke recovery; neurogenesis and neuroplasticity, and
thus figure out how to make those recovery signals repeatable'. But we have to do
human testing in stroke survivors first.
But the leaders would already have started listening to brain signals using one of these already. Add sarcasm tag here.
1. Use nanowires to listen in on single neurons
2. Or lay a grid across the cortex
to listen in.
But we have NO stroke leaders, nothing will get done until we get survivors in charge.
Leaders solve problems, they don't run away from them.
Ask your competent? doctor if this is better for tracking neuronal signals than these other ones?
The latest here:
Months-long tracking of neuronal ensembles spanning multiple brain areas with Ultra-Flexible Tentacle Electrodes
Nature Communications volume 15, Article number: 4822 (2024)
Abstract
We introduce Ultra-Flexible Tentacle Electrodes (UFTEs), packing many independent fibers with the smallest possible footprint without limitation in recording depth using a combination of mechanical and chemical tethering for insertion. We demonstrate a scheme to implant UFTEs simultaneously into many brain areas at arbitrary locations without angle-of-insertion limitations, and a 512-channel wireless logger. Immunostaining reveals no detectable chronic tissue damage even after several months. Mean spike signal-to-noise ratios are 1.5-3x compared to the state-of-the-art, while the highest signal-to-noise ratios reach 89, and average cortical unit yields are ~1.75/channel. UFTEs can track the same neurons across sessions for at least 10 months (longest duration tested). We tracked inter- and intra-areal neuronal ensembles (neurons repeatedly co-activated within 25 ms) simultaneously from hippocampus, retrosplenial cortex, and medial prefrontal cortex in freely moving rodents. Average ensemble lifetimes were shorter than the durations over which we can track individual neurons. We identify two distinct classes of ensembles. Those tuned to sharp-wave ripples display the shortest lifetimes, and the ensemble members are mostly hippocampal. Yet, inter-areal ensembles with members from both hippocampus and cortex have weak tuning to sharp wave ripples, and some have unusual months-long lifetimes. Such inter-areal ensembles occasionally remain inactive for weeks before re-emerging.
Similar content being viewed by others
Introduction
Complex behaviors such as perception, decision-making, and learning involve the interaction of many brain areas1,2. Techniques to perform long-term recordings of brain activity at single-cell resolution simultaneously from distributed brain areas are essential to investigate the coordinated brain dynamics underlying the learning and execution of such behaviors. Such high-resolution recordings can also improve our understanding of brain disorders and enable the development of new diagnostics and treatments that would be otherwise combinatorially impossible to discover using behavioral readouts alone3. However, currently available technologies for such recordings are limited, both in terms of their long-term stability and their applicability to multi-areal recordings. Although optical methods enable stable recordings of single-cell activity4, they cannot reach deep brain areas such as the hippocampus or thalamus without damaging the intermediate brain areas and require compromises between the number of cells recorded and temporal resolution5. They also require genetic modifications or the delivery of fluorescent indicators to neurons, making them impractical to use in the human brain.
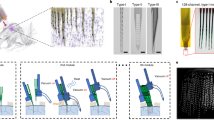
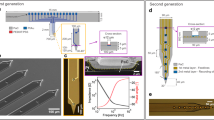
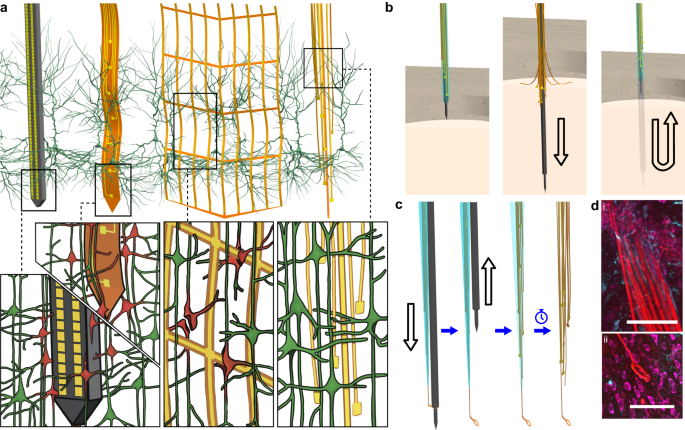
Extracellular electrode arrays, on the other hand, are typically fabricated on stiff materials and with large footprints that do not comply with the brain tissue, leading to glial encapsulation of the electrode arrays, loss of single units, low unit yields, drifts in recordings, and the inability to track single units across sessions6,7,8,9,10, unless ultra-high-density contacts are used to compensate for drifts and instabilities11,12. Flexible polymer electrode arrays have emerged as alternatives to stiff probes, offering superior biocompatibility, adaptability to the mechanics of the brain tissue, and stability of recordings13,14,15,16,17. However, recording from multiple brain areas with high-density flexible electrode arrays at cellular scale (<10 μm) without limitations on implantation depth while minimizing the implants’ footprints still remains a major challenge. Integrating more recording contacts on polymer shanks requires them to be wider, which leads to cutting through more neuronal structures during insertion (Fig. 1a). While this can be mitigated by distributing the recording contacts into independent tiny electrode fibers, inserting many such fibers simultaneously has been challenging due to their high flexibility and the shortcomings of the chemical glues to keep them together (Fig. 1b). For instance, polyethylene glycol, a water-soluble material commonly used for tethering electrode arrays to shuttles, can be dissolved in seconds inside the brain or even near the brain surface due to the humidity. Achieving stiffness or stability for insertion by using even more adhesive alone leads to larger insertion footprints. If, instead, a stiff insertion shuttle is used to achieve stiffness while holding together all the fibers and shuttle using a strong glue, the electrode fibers often get pulled out during retraction of the stiff insertion shuttle (Fig. 1b).
a Drawn-to-scale comparison of the geometries of a rigid silicon probe, flexible planar shank probe, neural mesh, and UFTE (from left to right) with reference to the soma and dendrites of surrounding neurons. Zoomed insets (bottom) show the impact of different probe geometries on neuronal processes after insertion into the brain. b Potential failures during the insertion of ultra-flexible electrode arrays coupled to a stiff shuttle purely by chemical tethering (left). The electrode fibers can separate prematurely from the stiff shuttle if the coating dissolves too fast (middle), or they can get stuck to the shuttle and be pulled out of the brain during shuttle retraction if the coating dissolves too slowly (right). c Delivery of the UFTE bundle into the brain, where the bundle is inserted into the brain with the help of a tungsten shuttle, the shuttle is retracted, and the silk coating is dissolved, leaving the electrode fibers independent from each other. d UFTE 3.5 months post-implantation. (i) UFTE bundle (red) shown with stained neurons (magenta) and the microglia (cyan). (ii) Loop of UFTE bundle in the same brain slice. Scale bars are 100 μm.
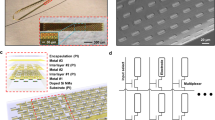
To overcome these issues, we developed Ultra-Flexible Tentacle Electrode (UFTE) arrays, which provide stable recordings of single units with exceptionally high mean signal-to-noise ratios (SNRs) of single-unit spikes, 1.5–3 times larger than state-of-the-art flexible electrode arrays15,16, with some mean single-unit SNRs as large as 89. We demonstrate that UFTEs can be inserted at least 6.5 mm deep from the dorsal surface while achieving high single-unit yields per recording contact. We also developed a technique to insert UFTEs simultaneously into many brain areas at arbitrary locations without any depth and angle-of-insertion limitations (Fig. 2d, e) to vastly simplify distributed recordings with UFTEs.
More at link.
No comments:
Post a Comment