Hasn't your competent? doctor put together a protocol on restoring your gut microbiota to decent levels to get you recovered from your stroke? NO? So, you DON'T have a functioning stroke doctor, do you?
gut microbiota
(33 posts to June 2016)
gut microbiota dysbiosis
(2 posts to March 2022)
gut microbiota transplants
(1 post to October 2023) You need to be aware of this problem.
Association of gut microbiota and immunometabolic profiles with ischemic stroke severity
Scientific Reports volume 15, Article number: 14046 (2025)
Abstract
This study investigates the influence of three regulators of human homeostasis—intestinal microbiota, immune profile, and circulating metabolites—on stroke severity. We conducted a study involving 33 patients with mild/moderate stroke (MS) and 32 with severe stroke (SS), all assessed during the acute phase (first 24 h). The analysis focused on microbiota composition (45 patients), serum metabolomics and inflammatory markers (20 patients). The patients with SS exhibited more pronounced insulin resistance associated with increased levels of branched-chain amino acids and their metabolites. SS patients showed an increase in inflammatory cytokines IL-6 and TNF-α, and surprisingly an increase in IL-10 and butyrate which are anti-inflammatory. SS patients also displayed a distinct microbiota profile, with statistically significant differences in β-diversity compared to the MS group, notably a higher prevalence of Pseudomonadota (formerly Proteobacteria). In summary, our data indicate that patients with SS, compared to those with MS, are characterized by a more inflammatory and insulin-resistant state, associated with three key regulators: microbiota, metabolites, and interleukins. These findings provide new insights into the regulatory components of the gut-brain axis, which could be developed into cost-effective and widely accessible therapies for SS.
Introduction
Stroke is one of the most prevalent cardiovascular diseases worldwide, being the second leading cause of death and a major cause of disability1,2. Approximately 12.2 million people experience new stroke episodes each year, with one occurring every three seconds, according to the World Stroke Organization. In the United States, around 800,000 people suffer from strokes annually, with 87% of cases being cerebral ischemic strokes (CIS)3. This results in a cost of over 70 million dollars per year. Considering that more than 90% of risk factors are preventable, stroke remains a significant global public health issue3,4.
Recent advancements in the fields of metagenomics and metabolomics have shed light on the significant role of the gut microbiota in regulating neuroinflammation, behavior, and cognitive function through the complex microbiota-gut-brain axis. This axis involves a bidirectional communication system that incorporates both neuronal and non-neuronal mechanisms. As individuals age, there is a noticeable shift in the gut microbiome composition, characterized by decreased bacterial diversity, lower levels of beneficial bacteria, and an increase in pathogenic and opportunistic bacteria5,6. Numerous studies have linked the composition of the gut microbiome to overall health and longevity, highlighting the importance of maintaining a balanced microbiome5,6. Previous data showed that intestinal microbiota composition can influence the development and severity of ischemic stroke7,8,9,10,11,12. On the other hand, following a stroke, there are observable changes in gut microbiota composition, leading to compromised gut integrity, increased bacterial translocation, and alterations in host-microbe metagenomic pathways9,13,14,15. Metabolites produced by the gut microbiome, such as short-chain fatty acids, play a crucial role in regulating inflammation in both the peripheral and central nervous systems9,15. Disruption of the gut microbiota following a stroke, particularly the decrease in beneficial bacterial strains, can impact the immune system in the small intestine, resulting in an imbalance of immune cells and altered antigen presentation9,13,15. Understanding these changes in the gut microbiome and metabolomic shifts post-stroke could pave the way for innovative therapeutic interventions aimed at restoring gut homeostasis and improving patient outcomes.
Furthermore, a gap remains in the research regarding integrated data on the differences in intestinal microbiota, immune profiles, and circulating metabolites between MS and SS cases. The aim of this study is to compare the integrated response of the three regulators of human homeostasis—intestinal microbiota, immune profile, and circulating metabolites—between patients with MS and those with SS. This will provide valuable insights into the potential differences in gut health and microbiota-immune-metabolic profiles following strokes of varying severities.
Results
Characteristics of the participants.
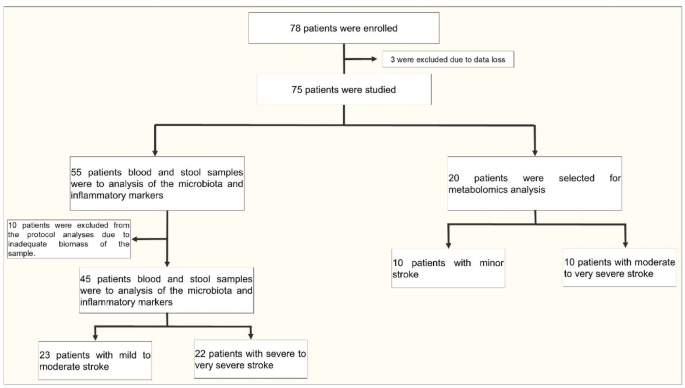
A total of 78 participants were enrolled, and 13 were excluded from the analysis, as described in the study design and population. This resulted in 65 participants in the whole study. Baseline demographic and clinical characteristics were similar in both groups (MS × SS) (Table 1).
In 45 participants (23 with MS and 22 with SS), we investigated the microbiota composition. In the other 20 participants (10 with MS and 10 with SS), we performed metabolomics and immunological profiling. The baseline demographic and clinical characteristics of this latter subgroup of 20 patients were very similar when comparing the 10 patients with MS to the 10 with SS (age: MS: 67.6 ± 11.6 years, SS: 66.1 ± 8.0 years; BMI: MS: 27.7 ± 3.7, SS: 27.6 ± 3.2).
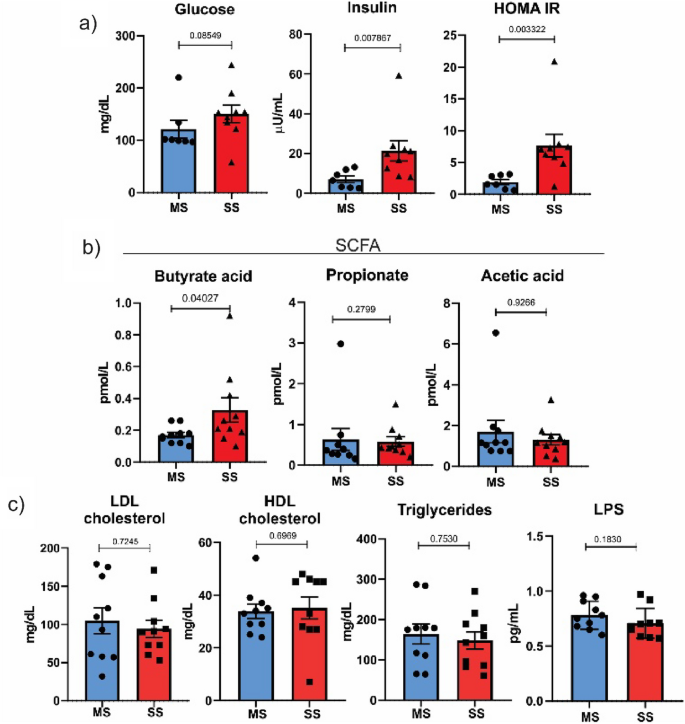
Plasma levels of glucose, insulin, SCFA and lipid profile
Figure 1 shows that plasma glucose levels were similar between patients with MS and SS. It is well known that insulin resistance is a risk factor for ischemic stroke, and then we determined insulin levels in the two groups of patients with ischemic stroke. The results showed that insulin levels were higher in the SS group, indicating a possible insulin resistance in this group. Reinforcing this data, we calculated HOMA-IR, which is a well-accepted index of insulin resistance, and the results showed an increased HOMA-IR in the SS group, confirming the insulin resistance of this group (Fig. 1).
Plasma levels of glucose, insulin, SCFA and lipid profile. (A) The HOMA-IR was calculated using the formula: HOMA-IR = (G × I)/22.5. (B) Serum concentrations of short-chain fatty acids determined through metabolomics analysis. (C) Serum concentrations of LDL cholesterol, HDL cholesterol, triglycerides and LPS determined through enzymatic methods. Results are presented as mean ± SD.
Butyrate, a short-chain fatty acid, was higher in the SS group compared to the MS group. The levels of acetate, propionate, and LPS did not show statistically significant differences between the two groups. The serum concentrations of LDL cholesterol, HDL cholesterol and triglycerides did not show statistically significant differences between the two groups (Fig. 1c).
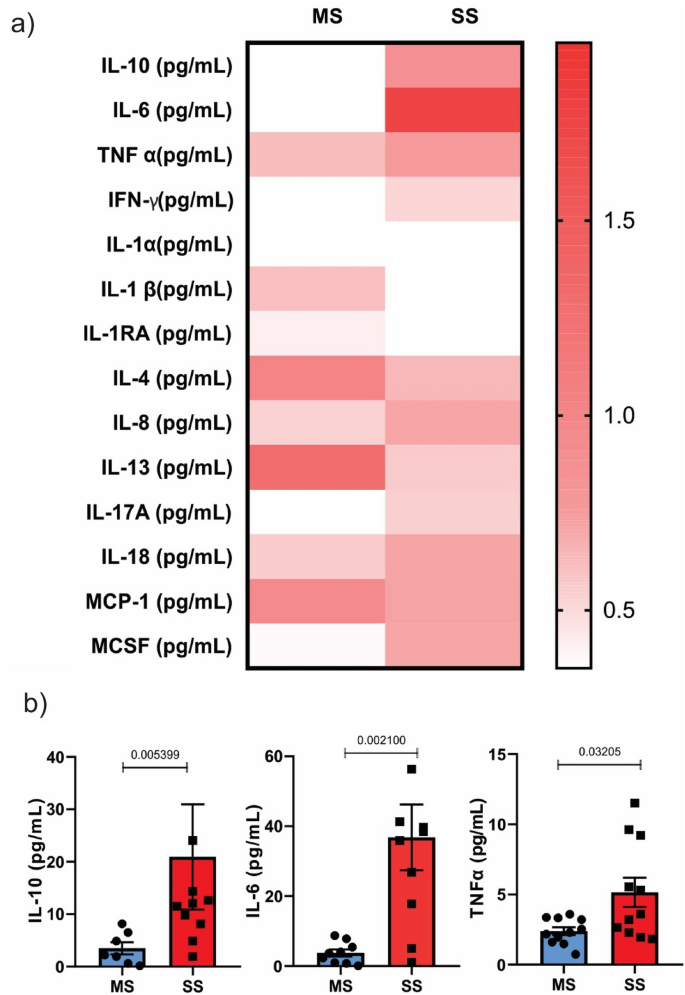
To investigate the inflammatory pattern of our patients, we determined the following interleukins: IFN gamma, IL-1 alpha, IL-1 beta, IL-1RA, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, IL-18, MCP-1, MCSF, and TNF alpha. The results showed that most of these interleukins were similar between groups, but three of them, IL-6, TNF alpha, and IL-10, were higher in patients with SS compared to MS patients (Fig. 2).
Intestinal microbiota in patients with acute ischemic stroke of different severity
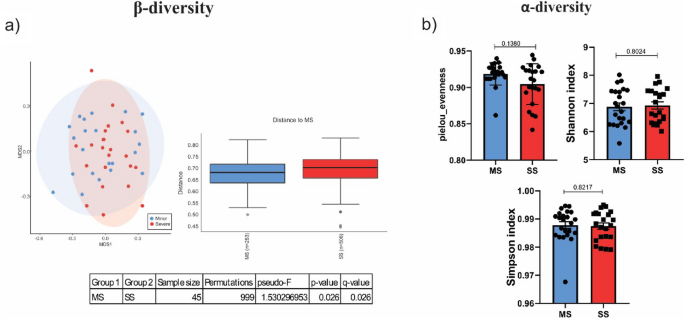
The composition of microbiota was investigated in 45 patients, 23 from MS and 22 from SS group. The NMDS plot based on Bray–Curtis dissimilarity suggests differences in the gut microbiota composition between the MS and SS groups. The UniFrac metric is a measure of β-diversity that assesses the phylogenetic distance between microbial communities, considering both the presence or absence of species and their evolutionary relationships. A Pseudo-F value of 1.53 indicates a moderate difference between the groups being compared (p-value of 0.026 and q-value of 0.026). Alpha-diversity is represented by Pielou’s Evenness, which measures the uniformity of species distribution in a microbial community. Higher values indicate a more even distribution of species. Although the SS group presented a lower Pielou’s Evenness index and these values were less homogeneous among the samples within the group, the difference was not statistically significant compared to the MS group (ƿ -value of 0.1380). Similarly, the Shannon and Simpson diversity indices did not reveal statistically significant differences between the groups, suggesting that the overall diversity within each group remains comparable (Fig. 3).
Diversity measure. (A) Beta diversity. The beta diversity based on Bray–Curtis dissimilarity is presented in a Non-metric Multidimensional Scaling (NMDS) plot showing the microbiota dissimilarity between groups. UniFrac metric measures the phylogenetic distance between sets of microbial communities (MS vs SS. Pseudo-F value of 1.53; ƿ-value of 0.026 and q-value of 0.026). (B) Alpha-diversity represented by the Pielou’s Evenness (ƿ-value of 0.1380).
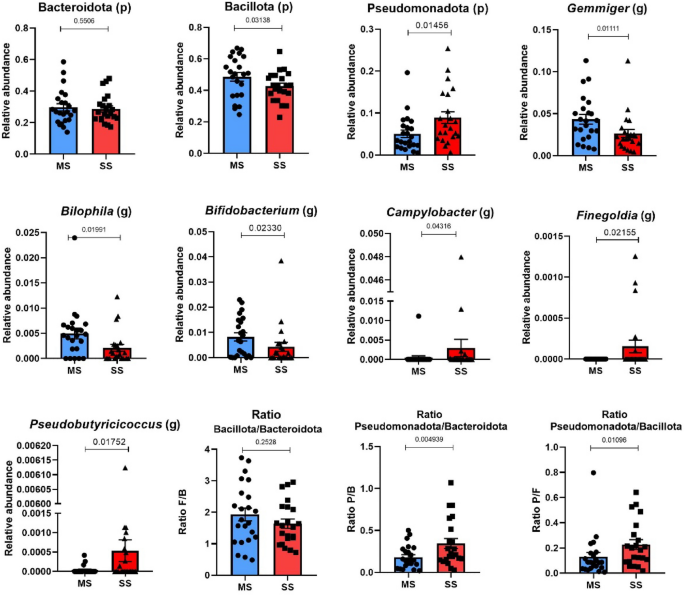
Taxonomic composition of community through direct quantitative comparison of abundances of phyla and genus. At the phylum level Pseudomonadota (formerly Proteobacteria) (ƿ-value 0.01456) was enriched in SS compared to MS. The genera Bilophila (ƿ-value 0.0199), Gemmiger (ƿ-value 0.0111) and Bifidobacterium (ƿ-value 0.0233) were lower in SS and the genus Campylobacter (ƿ-value 0.0431), Finegoldia (ƿ-value 0.0215) and Pseudobutyrucucoccus (ƿ-value 0.0175) were all higher in SS compared to MS patients (Fig. 4). Project Accession: PRJNA1171778.
Taxonomic composition. Comparison of phylum and genus abundances between the SS and MS groups. At the phylum level, Pseudomonadota was higher in SS (ƿ-value 0.0136). At the genus level Gemmiger (ƿ-value 0.011), Bilophila (ƿ-value 0.0119) and Bifidobacterium (ƿ-value 0.023) were higher in MS Campylobacter (ƿ-value 0.0431), Finegoldia (ƿ-value 0.021) and Pseudobutyricicoccus (ƿ-value 0.0175) were higher in SS. (p) = phylum and (g) = genus. Results are presented as mean ± SD.
Metabolomics
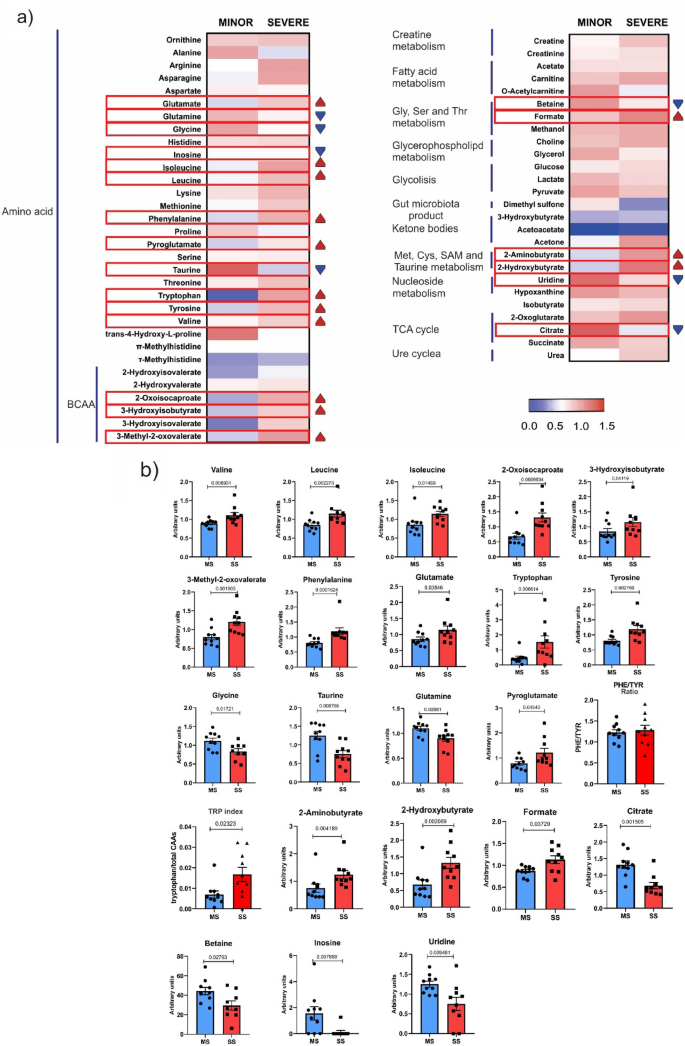
We performed metabolomic analysis in 20 patients—10 from the MS group and 10 from the SS group. The results showed that the branch chain amino acids valine, leucine, and isoleucine, along with their metabolites 2-oxoisocaproate, 3-hydroxybutyrate, and 3-methyl-2-oxovalerate, were significantly higher in patients with SS. The levels of phenylalanine, glutamate, tryptophan, and tyrosine were also significantly higher in these patients. On the other hand, the levels of glycine, taurine, and glutamine were lower in patients with SS, but the metabolite of glutamine, pyroglutamate, was higher in patients with SS. Previous data showed that the ratio of phenylalanine to tyrosine (PHE/TYR) was significantly higher in acute ischemic stroke (AIS) patients than in healthy controls16. Our data did not show differences between groups in the ratio of PHE/TYR. We also investigated another amino acid index, the tryptophan index, which is the ratio of tryptophan to its competing amino acids (CAAs) in circulation. This index reflects the relative availability of the essential amino acid tryptophan in the brain and is inversely associated with the risk of ischemic stroke. Surprisingly, our data showed that the tryptophan index was higher in patients with SS (Fig. 5).
Metabolomics Analysis. (A) Heat map illustrating metabolomic differences between the MS and SS groups. The heat map displays differential metabolites identified through metabolomics analysis and subsequent metabolic pathway analysis. The metabolites and their primary classes are listed on the left side of each row. Blue indicates a decreasing trend, while red represents an increasing trend. (B) Bar graph representing metabolites that showed statistically significant differences (values represented in arbitrary units) The TRP index was significantly higher in acute ischemic stroke (AIS) SS group compared to MS. No significant differences in the PHE/TYR ratio were observed between the study groups. Results are presented as mean ± SD.
The following metabolites were also significantly higher in patients with SS compared to MS: 2-aminobutyrate, 2-hydroxybutyrate, and formate. In patients with SS, there was a reduction in the levels of citrate, betaine, inosine, and uridine.
Since we used two different subgroups to analyze microbiota and immunological/metabolomics profiles, we could only establish an indirect relationship between the general data obtained from the microbiota analysis and the general findings from metabolomics and inflammatory factors, as the patients from both subgroups share the same clinical characteristics, which was a limitation of our study. On the other hand, it was possible to correlate data from metabolomics with the immunological profile, which were data from the same subgroup. The results showed positive correlations between TNF alpha and formate (r = 0.59, p-value 0.006); TNF alpha and valine (r = 0.46, p-value 0.037); and between IL-6 and phenylalanine (r = 0.54, p-value 0.016). There were also negative correlations between IL-6 and inosine (r = -0.62, p-value 0.005) and between IL-6 and uridine (r = -0.50, p-value 0.028).
Discussion
The results of the present study showed that patients with SS have a more pronounced inflammatory state and more severe insulin resistance compared to those with MS. This was associated with responses involving the intestinal microbiota, metabolites, and immune profile, which may play a determining role in this inflammation. Insulin resistance, a tissue marker of inflammation in SS, was characterized not only by elevated fasting insulin levels and HOMA-IR but also by other markers, such as increased circulating branched-chain amino acids and their metabolites—2-oxoisocaproate, 3-hydroxybutyrate, and 3-methyl-2-oxovalerate—which are associated with metabolic and cardiovascular morbidities17,18,19. In patients with SS, the levels of oxidative stress-related metabolites, 2-aminobutyrate and 2-hydroxybutyrate20,21, were also higher than in those with MS, indicating greater oxidative damage. These conditions, along with increased inflammatory cytokines, contribute to insulin resistance in SS patients. In accordance, there was a positive correlation between valine (BCAA) and TNF alpha, suggesting that multiple and associated mechanisms contribute to insulin resistance in SS patients.
Our data also confirms previous findings that correlated certain circulating amino acids, which are not directly related to insulin resistance, with prognosis in ischemic stroke. Zhu et al.22 demonstrated that specific plasma amino acid neurotransmitter dysregulation (increased glutamic acid, aspartic acid, and gamma-aminobutyric acid, and decreased glycine in plasma) may be implicated in the development and adverse outcome after ischemic stroke. In accordance, our data showed that the levels of glutamate were increased, and the levels of glycine were decreased in the group of patients with SS. Previous data have shown that decreased glutamine and increased glutamate during a stroke lead to an influx of calcium, which triggers ROS production and excitotoxicity23 leading the dying neurons to activate cytokines via microglial activation24. The reduction in taurine levels in SS patients aligns with previous studies, which reported a significant inverse association between serum taurine and stroke risk among never-smokers25, as well as a decrease in taurine levels in patients with ischemic stroke26. Unexpectedly, the tryptophan index, which is inversely associated with the risk of ischemic stroke, was higher in patients with SS. A reduced TRP index in patients with acute ischemic stroke indicates lower bioavailability of TRP for 5-HT synthesis in the brain of these patients27,28. Our data showing an increase in this index in patients with SS may reflect a counter-regulatory mechanism attempting to protect the brain from ischemic stroke by increasing the bioavailability of TRP in these patients.
Another interesting data is a reduction in circulating levels of the metabolite’s betaine and inosine in patients with SS. Although not completely understood, the reductions in circulating levels of inosine and betaine in patients with SS stroke may reflect reduced ingestion, reduced absorption, modulation by microbiota that produce inosine, or reduced production by the body. The influence of a more inflammatory state presented by these patients in these processes deserves further investigation. It is important to mention that BCAA, which is increased in SS patients, can reduce circulating levels of uridine29, contributing to explaining, at least in part, the reduced uridine levels in these patients. It should be emphasized that there are data showing that higher levels or supplementation of diet with these metabolites can improve neurologic diseases, including stroke30,31,32. The reduction in nucleoside uridine in SS patients is also in accordance with previous data that showed that in two cohorts, uridine was inversely related to stroke risk33. In accordance, there was a negative correlation between IL-6 and inosine or uridine.
The circulating cytokines exhibited a pattern characterized by an increase in the inflammatory markers IL-6 and TNF-alpha. Surprisingly, patients with SS also showed an increase in IL-10, an anti-inflammatory cytokine, which warrants further discussion. This increase in IL-10 may be attributed to the small cohort size in our study, along with potential imbalances between the two groups, such as the use of beta-blockers, insulin, and metformin in the MS group. However, it may also represent a compensatory response to the inflammatory profile34. There is clear evidence that higher circulating IL-6 and TNF alpha levels in community-dwelling individuals are associated with higher long-term risk of incident ischemic stroke independently of conventional vascular risk factors35. In accordance with our data Waje-Andreassen et al. showed that IL-6 levels were increased in the acute phase of stroke compared with healthy controls and correlated with larger stroke volume, indicating that IL-6 seems to be a robust early marker for outcome in acute ischemic stroke36. On the other side, previous data, reinforcing our results, showed that IL-10 deficient mice exhibit significantly increased infarct sizes on days 3 and 7 and enlarged brain atrophy and impaired neurological outcome on day 14 following a model of ischemic stroke by transient middle cerebral arterial occlusion, reinforcing the protective role of IL-1034. The increase in IL-10 may also help explain the elevated TRP index in SS patients, as IL-10 may exert a neuroprotective effect and prevent the potentially reduced capacity for 5-hydroxytryptamine synthesis in the brain37. Taken together with previous data, we can suggest that the increase in IL-10 following the onset of the stroke could be a potential direction for future studies.
The intestinal microbiota of patients with SS showed clear differences from those with MS stroke, displaying signatures commonly associated with insulin resistance. Our results indicated that β-diversity, but not α-diversity, exhibited statistically significant differences between the SM and SS groups. Similarly, the β-diversity of gut microbiota in participants from the Rotterdam Study was linked to insulin resistance38. Previous studies showed that the phylum Pseudomonadota was significantly more abundant in patients with ischemic stroke. Our data further reveal that Pseudomonadota was more prevalent in SS compared to MS patients. The outer membrane of Pseudomonadota contains lipopolysaccharides (LPS), which can induce metabolic endotoxemia, an inflammatory condition leading to insulin resistance39,40. However, LPS levels were very similar in patients with SS compared to those with MS disease, suggesting that LPS likely does not explain the differences in insulin resistance between the groups. Nonetheless, it is important to consider that after LPS translocation from the gut, its primary site of action is the liver. In a previous study, we demonstrated that portal LPS levels might better reflect its translocation than circulating peripheral levels41. Therefore, we cannot entirely exclude the possibility that LPS contributes to hepatic insulin resistance in patients with SS.
Finegoldia magna, a Gram-positive anaerobic coccus, expresses FAF and L proteins that activate neutrophils and induces NET (neutrophil extracellular trap) formation and immune responses. FAF neutralizes antimicrobial peptides, while protein L promotes inflammation and vascular leakage. Our data show that genus Finegoldia from the intestinal microbiota is enriched in patients with SS. Considering that F. magna modulates the immune response by inducing inflammation and NETs, it may contribute to systemic inflammation, metabolic dysregulation, and insulin resistance observed in SS patients. The interplay between F. magna, gut microbiota, and immune responses warrants further investigation in the context of stroke pathophysiology and metabolic complications.42.
Other alteration in intestinal microbiota in patients with SS were also observed. The increase in Campylobacter was previously described in association with hyperglycemia/glucose intolerance and atherosclerotic cardiovascular disease, respectively43. Jie et al.44 performed a metagenome-wide association study on stools from 218 individuals with atherosclerotic cardiovascular disease (ACVD) and 187 healthy controls.
Previous data showed that probiotic supplementation with specific Bifidobacterium strains is able lower plasma levels of TMAO and trimethylamine (TMA), both of which are associated with cardiovascular diseases45,46. Our data showing that the genus Bifidobacterium was decreased in the SS group may suggest that these patients are less protected from cardiovascular diseases and stroke.
The reduction in the percentual of the genus Gemmiger is relevant for cardiometabolic diseases. Ray et al47 demonstrated reduced levels of the genera Gemmiger in cardiometabolic diseases and in intestinal inflammatory diseases, probably linked to inflammatory states and altered gut permeability.
Although intestinal microbiota influences the prognostic in ischemic stroke, there is recent data showing that opposite occurs, with stroke causing dysregulation of intestinal microbiota. It is possible that this microbiota modulation by stroke may be a defense mechanism, to protect against the inflammatory burden. A recent study revealed that patients with cerebral ischemic stroke (CIS) had a higher prevalence of SCFA producers, including Odoribacter, Akkermansia, Oscillospiraceae UCG-005, and Victivallis7. In our study, we observed an increase in the genus Pseudobutyricicoccus in patients with SS. This genus was first proposed in 2020 by Glendinning et al. within the order Oscillospirales recognized for its butyrate production48,49. Additionally, circulating levels of butyrate were found to be elevated in patients with SS. In this regard, our data reports a contradictory finding to previous studies in terms of butyrate50,51,52. Although the reasons for this difference are not well established, some possibilities should be considered. One important point is that our study did not compare stroke patients with individuals without stroke; rather, we compared different levels of severity among stroke patients. Additionally, some previous studies that reported a negative correlation between butyrate and stroke outcomes were conducted in animal models53,54,55. Other important differences may be related to the different populations investigated and experimental methods, such as the determination of butyrate in feces in previous studies50. On the other hand, this increase in butyrate may be a secondary compensatory response to the marked inflammatory state. This hypothesis is consistent with previous findings indicating that higher levels of SCFAs at the time of stroke are associated with increased markers of inflammation15. This elevation is closely linked to increased levels of inflammatory markers that contribute to stroke pathology. Elevated plasma SCFA levels may serve as a pathological factor in the acute dysregulation of intestinal microbiota9. SCFAs are transported from the gut into the plasma, where they can directly influence peripheral immune responses and indirectly modulate intracranial immune responses9. Taken together, our results suggest that the inflammatory response that helps establish, maintain, and/or is triggered by severe stroke may be modulated, at least in part, by the microbiota through increased butyrate production and by the immune system through elevated IL-10 production, which may also influence the TRP index.
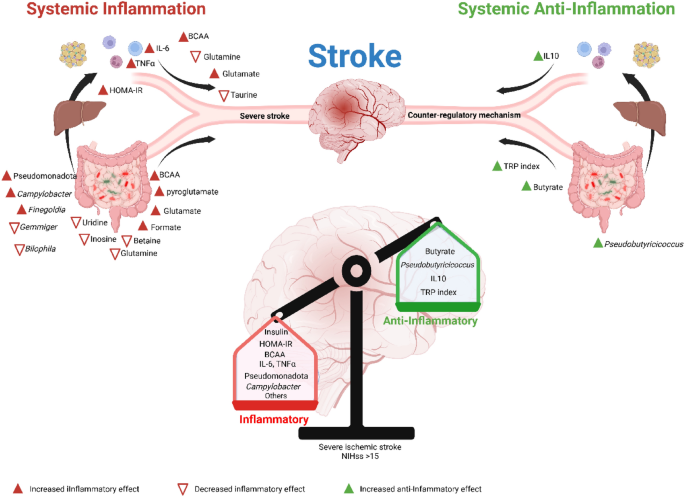
In summary, our data showed that patients with SS, compared to those with MS, are characterized by more inflammatory and insulin-resistant states, accompanied by three main regulators: microbiota, metabolites, and interleukins, and associated with an anti-inflammatory response, but it is insufficient to overcome the inflammatory state. By demonstrating that inflammation in SS may have compared from different sources, our findings help elucidate novel regulatory components of the gut-brain axis, which could be developed into cost-effective and widely accessible therapies for severe ischemic stroke. A visual summary of the study’s main findings is provided in Fig. 6.
Graphical Abstract. Severe ischemic stroke, compared to mild/moderate stroke, is characterized by more pronounced insulin resistance, along with alterations in the microbiota, metabolites, and interleukins. These changes reflect an inflammatory state; however, they may also indicate the presence of a potential anti-inflammatory defense mechanism. We suggest that the balance between these opposing responses, partly influenced by the microbiota, could play a role in determining the severity of the stroke.
Methods
Study design and population
Patients with acute ischemic stroke (AIS) were recruited for this cross-sectional observational study. Initially, individuals with suspected AIS were admitted to Hospital Estadual Sumaré (HES) at the University of Campinas (UNICAMP) between February 2018 and December 2022. A non-contrast CT scan was performed to confirm the diagnosis. Those with confirmed stroke were interviewed and asked to provide informed consent. Patients who consented had fresh blood and/or stool samples collected, which were transported on ice packs to the Laboratory of Clinical Investigation in Insulin Resistance (LICRI) at UNICAMP for further analysis. The samples were processed to assess microbiota, inflammatory markers, and metabolomics. Patients who did not provide consent were excluded from the study. Additionally, none of the participants had gastrointestinal diseases or had taken antibiotics or probiotics for at least one month prior to biospecimen collection. The study and all experimental protocols were approved by the Ethics Committee of UNICAMP, under the approval number CAAE (72318317.3.0000.5454) and were conducted in accordance with relevant guidelines and regulations. All patients or their legal guardians involved in this study provided written informed consent.
This cross-sectional observational study involved patients diagnosed with ischemic stroke. A total of 78 patients were initially enrolled, but 3 were excluded due to data loss, leaving 75 patients for analysis. The study focused on two key areas: the evaluation of microbiota and inflammatory markers, and metabolomics analysis. For the microbiota and inflammatory markers analysis, blood and stool samples were collected from 55 patients. However, 10 patients were excluded due to insufficient sample size, resulting in 45 patients eligible for further evaluation. These patients were categorized based on stroke severity, using the NIH Stroke Scale (NIHSS). Patients with a score of up to 14 were classified as having MS, while those with a score greater than 15 were classified as having SS. This resulted in 23 patients with MS and 22 patients with SS. Simultaneously, 20 patients were selected for metabolomics analysis. These patients were divided into two groups: 10 with MS and 10 SS (Fig. 7). This design enabled a detailed comparison of microbiota composition, inflammatory markers, and metabolomic profiles across various levels of stroke severity.
Serum dosage of LPS and interleukins
Serum samples were obtained from blood samples and diluted to 20% (vol./vol.) with endotoxin-free water and then heated at 70ºC for 10 min to inactivate serum proteins. Then, LPS was quantified using a commercial Limulus Amebocyte Assay kit (Cambrex, Walkersville, MD, USA) according to the manufacturer’s protocol, as previously described41.
To quantify human insulin levels in serum, we used the Sandwich ELISA method with a non-radioactive kit from Merck (EZHI-14K). Circulating glucose levels were measured using the glucose oxidase method.
Samples were aliquoted for multiplex immunoassay (Bio-Plex 200; Bio-Rad Laboratories, Hercules, CA), which analyzed magnetic bead panels for : IFN gamma, IL-1 alpha, IL-1 beta, IL-1RA, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, IL-18, MCP-1, MCSF and TNF alpha (HCYTOMAG-60K and HMMP2MAG-55K-01, MILLIPLEX MAP Human; MilliporeSigma, Merck KGaA).
Short-chain fatty acid analysis (SCFA)
The collected samples were centrifuged (Eppendorf 5804R, 4000 g, 10 min, 4 °C) and filtered through a cellulose acetate membrane (0.45 μm porosity, Chromafil Xtra CA-45/5, Duren, DE) to remove suspended solids. Then, NaCl (1 g), crotonic acid (100 μL), isobutanol (70 μL), and 2 M H2SO4 (200 μL) were added. Analytical curves were constructed from stock solutions of the acids of interest (acetic, propionic, and butyric acids). SCFA analysis was conducted using an HP-6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a split/splitless injector and an HP-5975 mass detector (Agilent Technologies, Santa Clara, CA, USA).
Quantification of metabolites
The metabolites were processed and quantified using NMR Suite software version 8.1 (Chenomx Inc™, Edmonton, AB, Canada). The processor module of this software was used to adjust the spectral phase and perform baseline corrections. A 0.5 Hz line-broadening function was applied to reduce signal noise and facilitate the fitting of the metabolite signals into spectral peaks. The water signal was suppressed, and the spectra were calibrated using the reference signal of TMSP-d4 at 0.5 mM. The spectra were then individually transferred to the Profiling module of the software to determine the metabolomic profile of each group. Metabolites were identified, and their concentrations were measured. Metabolite concentration data were exported to Excel® (Microsoft Office™ 365) and absolute quantification was performed, eliminating the need for any normalization of the reported data56. The values represented in arbitrary units were obtained by dividing each value by the total average of each metabolite.
We also calculated the tryptophan index (TRP index), taking into account that during tryptophan transport from circulation via the large neutral amino acid transporter system, tryptophan competes with other amino acids (competing amino acids, CAAs), namely tyrosine, valine, phenylalanine, leucine, and isoleucine. The TRP index is calculated as the ratio of tryptophan to total CAAs.
Microbiota analysis
Patients’ total stool samples were collected on the same day of admission and were stored at − 80 °C until the analysis. Samples were collected, stored and processed in a controlled environment to minimize the risk of contamination. The genomic DNA from 200 mg of stool was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). A negative control (water from QIAamp DNA Stool Mini Kit) was used from an extraction step to the final sequencing and mock microbial DNA community standard was used as a positive control (ZymoBIOMICS Irvine, CA, USA). For each sample the V3–V4 hyper-variable region (Primer Forward TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG; Reverse GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) of the bacterial 16S rRNA gene was amplified followed by Illumina 16S Metagenomic Sequencing Library Preparation guide. The taxonomic composition of the bacterial communities was obtained by analyzing the V3–V4 region of the 16S rRNA gene using the Illumina® MiSeq platform. The constructions of the DNA sequencing libraries were performed according to the manufacturer’s instructions (Illumina, San Diego, CA, USA) and followed the same flow described by Caporaso et al. (2012). The fastq sequences were analyzed using the Illumina 16S Metagenomics software. The taxonomic classification was performed using the DADA2 pipeline and the GreenGenes database57,58 (version 2024.09). Paired abundance analyzes were performed using the IBM SPSS® 20.0 software (Wilcoxon Signed Ranks Test). The analysis of alpha (Metrics: Pielou’s evenness) and beta diversity (Metrics: Unweighted UniFrac, weighted UniFrac )was performed using QIIME59. The graphs were generated by GraphPad Prism 7.0 and Graphical Software: R (version 4.3.1).
Statistical analysis
Quantitative variables were presented as to their distribution pattern: mean and standard deviation (SD), or median, minimum, and maximum values. The Student’s t-test was used to compare two independent samples and a non-parametric (Mann–Whitney) test for variables without normal distribution. Categorical variables were presented as proportions, and we used either Pearson χ2 or Fisher’s exact tests to compare two proportions from independent samples. We utilized IBM SPSS Statistics for Windows, version 20.0. The level of significance adopted for the statistical tests was 5%.
Data availability
All the data produced or examined during this study are presented within this article. The 16S rRNA sequence dataset has been deposited in the BioProject repository (PRJNA1171778).
References at link.
No comments:
Post a Comment