Use the labels in the right column to find what you want. Or you can go thru them one by one, there are only 31,934 posts. Searching is done in the search box in upper left corner. I blog on anything to do with stroke. DO NOT DO ANYTHING SUGGESTED HERE AS I AM NOT MEDICALLY TRAINED, YOUR DOCTOR IS, LISTEN TO THEM. BUT I BET THEY DON'T KNOW HOW TO GET YOU 100% RECOVERED. I DON'T EITHER BUT HAVE PLENTY OF QUESTIONS FOR YOUR DOCTOR TO ANSWER.
What this blog is for:
My blog is not to help survivors recover, it is to have the 10 million yearly stroke survivors light fires underneath their doctors, stroke hospitals and stroke researchers to get stroke solved. 100% recovery. The stroke medical world is completely failing at that goal, they don't even have it as a goal. Shortly after getting out of the hospital and getting NO information on the process or protocols of stroke rehabilitation and recovery I started searching on the internet and found that no other survivor received useful information. This is an attempt to cover all stroke rehabilitation information that should be readily available to survivors so they can talk with informed knowledge to their medical staff. It lays out what needs to be done to get stroke survivors closer to 100% recovery. It's quite disgusting that this information is not available from every stroke association and doctors group.
Wednesday, December 17, 2025
Changes in Driving Patterns May Signal Early Cognitive Decline
Your competent? doctor already knew about this earlier research, right? Oh no, you DON'T have a functioning stroke doctor, do you?
And your patient's health will deteriorate after stopping driving.
Health goes downhill when older adults stop driving
The latest here:
Changes in Driving Patterns May Signal Early Cognitive Decline
A change in driving patterns may be an indicator of cognitive decline in older adults, a long-term study suggested.
Using driving data, researchers were able to predict the development of mild cognitive impairment (MCI) with 82% accuracy. Over 3 years, compared with those with normal cognition, older adults with MCI drove less at night, made fewer monthly trips, and did not stray as much from regular routes.
“We found that using a GPS [Global Positioning System] data tracking device, we could more accurately determine who had developed cognitive issues than looking at just factors such as age, cognitive test scores, and whether they had a genetic risk factor related to Alzheimer’s disease,” principal investigator Ganesh M. Babulal, PhD, OTD, of Washington University School of Medicine in St. Louis, said in a news release.
The study was published online on November 26 in Neurology.
New Opportunities for Early Detection
In the US, older adults make up roughly 20% of drivers. In addition, an estimated one third of this population experiences cognitive impairment. Previous studies have shown that those with early-stage dementia scored worse on driving tests and had a greater risk of a crash.
Timely, scalable solutions are needed to monitor safety in this at-risk population of drivers, the researchers noted, highlighting that recent advances in vehicular tracking technology might provide useful data to identify MCI.
To test this hypothesis, the investigators analyzed driving data from 298 participants (mean age, 75.1 years; 45.6% female). Of these, 56 were older adults with MCI, while the rest had normal cognition (NC).
Participants underwent the Clinical Dementia Rating, a series of neuropsychological assessments, and were genotyped for the APOE epsilon 4 allele, a known risk factor for Alzheimer’s disease. Investigators calculated individuals’ Preclinical Alzheimer Cognitive Composite (PACC) score based on results from a battery of standardized cognitive tests.
An in-vehicle GPS tracker recorded participants’ driving behavior daily for up to 40 months, capturing total trips, average distance, nighttime driving, speeding episodes, and route variation.
Changes in longitudinal driving behavior were assessed using a linear mixed model, adjusted for baseline age, race, education, sex, and APOE epsilon 4 status. Logistic regression with receiver operator curve analysis was used to distinguish older adults with MCI and those with NC.
At baseline, the driving habits of the NC and MCI groups were similar. However, over time, older adults with MCI made fewer trips per month (P < .001) and fewer nighttime trips (P < .001). They also drove more familiar routes, as evidenced by statistically significantly lower random entropy, a measure of trip unpredictability.
Specifically, driving factors like speeding, route variation, and medium and maximum distance were found to distinguish drivers with MCI from those with NC (area under the curve, 0.82; 95% CI, 0.75-0.89).
When demographic factors, PACC score, and APOE epsilon 4 status were added to the model, the investigators were able to identify MCI with 87% accuracy (95% CI, 0.81-0.93). Without the driving data, accuracy decreased to 76%.
“Looking at people’s daily driving behavior is a relatively low-burden, unobtrusive way to monitor people’s cognitive skills and ability to function,” Babulal said. “This could help identify drivers who are at risk earlier for early intervention, before they have a crash or near miss, which is often what happens now.”
The investigators noted that the study’s limitations included the fact that the majority of participants were predominantly White individuals and highly educated and that the data were not externally validated.
The study was supported by the National Institutes of Health and the National Institute on Aging. See the study for the full list of author disclosures.
High-Fat Cheese May Have Cognitive Benefit, Study Suggests
Your doctor has informed you of the benefits of dairy fat, right?
Maybe ask about Grana Padano cheese and see how long your doctor has been incompetent!
Study: Aged Cheese Lowers Blood Pressure September 2019
Eating cheese may offset blood vessel damage from salt Article no longer available so have your doctor find it.
Dairy fat from milk, butter, and cheese could actually PREVENT a heart attack September 2021
Oh no, your doctor is totally fucking incompetent? And you're paying them?
But a more nuanced analysis here:
The latest here:
High-Fat Cheese May Have Cognitive Benefit, Study Suggests
Key Takeaways
- A higher intake of high-fat cheese and cream was linked to lower dementia risk in a large Swedish study.
- Low-fat cheese, milk, and butter had no association with dementia risk over 25 years.
- The findings align with data from other studies, challenging long-held assumptions about fat and brain health.
Higher intake of high-fat cheese and cream in midlife was tied to a lower risk of subsequent dementia, a 25-year study in Sweden showed.
Adults who ate 50 g or more of high-fat cheese daily had a 13% lower risk of all-cause dementia (HR 0.87, 95% CI 0.78-0.97) and a 29% lower risk of vascular dementia (HR 0.71, 95% CI 0.52-0.96) compared with those who ate less than 15 g a day, said Yufeng Du, PhD, of Lanzhou University in China, and co-authors.
Those who consumed 20 g or more of high-fat cream daily had a 16% lower dementia risk compared with those who consumed no high-fat cream (HR 0.84, 95% CI 0.72-0.98), the researchers reported in Neurology.
Low-fat cheese, low-fat cream, and other dairy products, including milk (regardless of fat content), fermented milk, and butter, had no significant association with dementia risk.
High-fat cheeses have more than 20% fat and include cheddar, Brie, and Gouda; two slices of cheddar were roughly equal to 50 g. High-fat creams typically contain 30-40% fat and include whipping cream, double cream, and clotted cream.
This study was observational and cannot prove causality, noted co-author Emily Sonestedt, PhD, of Lund University in Sweden. "The most likely interpretation is that high-fat cheese was part of a broader dietary pattern that supported vascular health in this population," she told MedPage Today.
The study results "align with previous research showing that certain fermented dairy products are not harmful -- and may even be beneficial -- for cardiovascular health, which is closely linked to brain health," Sonestedt said.
Nonetheless, the outcomes challenge "some long-held assumptions about fat and brain health," she noted.
The data come on the heels of other research that suggested that the risks associated with high-fat foods may be overstated.
"For decades, public health advice has championed low-fat dairy, a recommendation born from concerns that the saturated fat in whole-fat products would elevate the risk of cardiovascular disease," wrote Tian-Shin Yeh, MD, MMSc, PhD, of Taipei Medical University in Taiwan, in an accompanying editorial.
"More recently, this view has been challenged by publications suggesting that dairy fat may have a neutral association with cardiovascular outcomes, reigniting the controversy," Yeh added. "When the focus shifts from the heart to the brain, the picture becomes even murkier."
The Swedish findings need to be replicated in other populations with varying dietary patterns, she noted.
"To move beyond association and toward causation, randomized trials should test the effects of specific dairy products, such as high-fat versus low-fat and fermented versus non-fermented options, as well as the impact of replacing dairy with high-quality plant-based alternatives on cognitive outcomes," Yeh wrote.
"While the development of dementia requires extended follow-up to capture, trials using cognitive decline or other intermediate outcomes may offer more feasible paths forward," she suggested.
Du and colleagues studied 27,670 participants in the prospective Malmö Diet and Cancer cohort who had baseline dietary assessments that involved a 7-day food diary, a food frequency questionnaire, and a dietary interview.
Participants had a mean baseline age of 58 years, and 61% were female. The researchers identified dementia diagnoses using Swedish National Patient Register data through 2020, validating cases before 2014.
The median follow-up was 24.9 years. During that period, 3,208 incident dementia cases were recorded, including diagnoses of vascular dementia and Alzheimer's disease. High-fat cheese intake was associated with a lower risk of Alzheimer's among APOE4 noncarriers, but not among carriers.
The study had several limitations, the researchers acknowledged. Unmeasured confounding may have influenced outcomes. Diet was assessed only at baseline and changes may have occurred over the follow-up period. The findings are based on Swedish people and may not apply to other populations.
"A major strength of our study is the detailed dietary assessment and validated dementia diagnoses, but we need similar studies in other populations with different eating habits," Sonestedt said.
"For clinicians, the key message is that moderate amounts of high-fat cheese do not appear detrimental to brain health and may fit within an overall healthy diet," she noted.
Social Isolation Directly Speeds Up Cognitive Decline
So, make sure your incompetent? doctor has EXACT 100% RECOVERY PROTOCOLS so you can go directly back to your old life and not lose chunks of friends due to your stroke,
Social Isolation Directly Speeds Up Cognitive Decline
Summary: Social isolation has a direct causal impact on how quickly cognitive function declines in later life, independent of whether someone feels lonely. By analyzing more than 137,000 cognitive tests from over 30,000 older adults, the study found that reduced social contact consistently predicted faster decline across every demographic group.
Loneliness and isolation each influence health, but only isolation reliably caused cognitive deterioration. With rates of Alzheimer’s already high and no cure available, the findings highlight that strengthening social connection is not only emotionally beneficial but neurologically protective.
Key Facts:
- Isolation as a Cause: Objective social isolation—not just loneliness—directly accelerates cognitive decline.
- Consistent Across Groups: The effect appeared across gender, race, ethnicity, and education levels.
- Large-Scale Evidence: Findings were based on over 137,000 cognitive assessments collected over 14 years.
Source: University of St. Andrews
New research from the University of St Andrews has discovered a direct causal effect between social isolation and a faster decline in later- life cognitive function. Pathological cognitive decline is most often driven by Alzheimer’s and related dementias
The study, published today (16 December 2025) in The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences found a consistent pattern of higher social isolation causing faster cognitive decline whether or not people identify as lonely.
Social isolation is objectively measured as, for example, sociability, membership in community organisations, and religious participation, whereas loneliness is a subjective report of how often people feel lonely.
Whilst social isolation and loneliness are often thought of as associated, they appear to have independent effects on cognitive function.
The School of Geography and Sustainable Development at St Andrews, alongside researchers from the Max Planck Institute for Demographic Research in Germany and Emory University in the US, analysed data from the US Health and Retirement study, looking at 137, 653 cognitive function tests taken between 2004 and 2018 by over 30 000 individuals.
They found that reducing social isolation has a protective effect on cognitive function for all subpopulations regardless of gender, race, ethnicity and educational level with only minor differences among social categories.
There has been a great deal of interest in the health effects of social isolation and loneliness, especially focussed on adolescents and older adults. Prior to Covid 19, about a quarter of older individuals (65 and over) identified as being socially isolated, spurring concerns about health implications. Likewise, loneliness has been recognised as a major public health issue in high income countries including the US, UK, Sweden, Australia, Germany and Japan.
In the US, Alzheimer’s disease already afflicts an estimated 6.9 million people, with around 1 in every 11 of over 65s effected in the UK. With no known cure, prevention is all important.
Lead author of the paper, Dr Jo Hale from the University of St Andrews, said: “Around the holidays, many of us think a lot about how important it is to be surrounded by family and friends. From Pagan winter holidays to classic Christmas tales, we’re reminded that social engagement is good for our mental health.
“This research shows that it’s also important for our cognitive health. As Alzheimer’s is a leading cause of death for older adults in the UK/US, constructing the scaffolding to allow for regular social interaction, especially for those who mayn’t have family or friends nearby, should be a public health priority.”
Key Questions Answered:
A: The study shows a direct causal link: higher isolation predicts faster decline regardless of subjective loneliness.
A: No. Loneliness is subjective and emotional, while isolation is behavioral; only isolation reliably drove cognitive decline.
A: Every subgroup studied—across all demographic lines—showed cognitive protection when isolation was reduced.
Editorial Notes:
- This article was edited by a Neuroscience News editor.
- Journal paper reviewed in full.
- Additional context added by our staff.
About this social isolation and cognition research news
Author: Ruth Sanderson
Source: University of St Andrews
Contact: Ruth Sanderson – University of St Andrews
Image: The image is credited to Neuroscience News
How the Mediterranean Diet May Slow Alzheimer Progression: Insights From Emerging Research
Pretty much useless, the Mediterranean diet has no specifics at all! Until further research tells us exactly what to consume, you're just hoping for the best. NOT GOOD ENOUGH!
How the Mediterranean Diet May Slow Alzheimer Progression: Insights From Emerging Research
Alzheimer Disease (AD) is a neurodegenerative disorder that affects memory, behavior, and cognition and is the leading cause of dementia.1 The underlying etiology involves the accumulation of beta-amyloid (Ab) and tau proteins, which contribute to brain atrophy and progressive cognitive decline. As of 2024, approximately 6.9 million Americans older than 65 years and 57 million adults worldwide were affected by AD.2,3 No cure exists for this disease, and current pharmacologic therapies target symptomatic relief only.3 This review examines the relationship between diet and dementia, with a focus on the role of a Mediterranean diet (MedDi) in potentially preventing or delaying cognitive decline in AD.
Pathophysiology of Alzheimer Disease
Several alterations in brain structure and function underlie the neuropathology of AD. The accumulation of Ab and tau proteins, neuronal loss, and atrophy are central features.4 Ab protein fragments aggregate extracellularly as plaques, and misfolded tau accumulates within neurons as neurofibrillary tangles. Ab plaques interfere with neuronal synaptic function, and tau tangles hinder internal cellular processes essential for neuron survival.2,4,5 These mechanisms result in neurodegeneration, synaptic loss, and chronic inflammation. Accelerated brain atrophy, beyond typical age-related volume decline, is a hallmark of AD, with additional contributions from oxidative stress and the disruption of gut microbiota composition.6,7
Risk Factors
AD is associated with both non-modifiable and modifiable risk factors. The most significant non-modifiable risk factor is advanced age, with prevalence doubling every 5 years after age 65.2,8 Other non-modifiable factors include family history, sex, and genetics.9 Key genetic influences include mutations in amyloid precursor protein, Presenilin-1 and -2, and apolipoprotein E.4,5 Although these factors are important, an estimated one-third of AD cases are attributable to modifiable risk factors, emphasizing an opportunity for prevention.3,9 Modifiable risks include type 2 diabetes (T2DM), atherosclerosis, cigarette smoking, obesity, hypertension, depression, decreased cerebral perfusion, lack of physical activity, and diet.3,4,9,10
Changes Occur Years Before Diagnosis
Neurodegenerative changes in AD typically begin up to 20 years before symptom onset, in the preclinical stage. Symptoms reflect the brain regions affected, especially those involved with memory, language, and executive function,11 and are classified as mild, moderate, or severe. Amnestic mild cognitive impairment (MCI), a precursor to AD, involves subtle cognitive changes but preserved functional independence; one-third of cases progress to mild AD within 5 years.2
Mild AD is characterized by mild memory loss, impaired judgment, mood changes, depression, diminished executive function, and difficulty managing finances.2,4 Individuals may require assistance with certain tasks, but largely function independently.2 In moderate AD, patients experience confusion; difficulty recognizing familiar faces; and trouble with reading, writing, speaking, and completing multi-step tasks.2,4 Severe AD, the final stage, manifests with motor dysfunction, speech and swallowing difficulties, profound memory loss, and reliance on full-time care. Physical complications, such as infections, are common.2
Significance of Prevention
Despite more than a century of research, no cure for AD has been identified.3,9,12 Pharmacologic regimens involving memantine, rivastigmine, galantamine, and donepezil provide only symptomatic benefit and minor improvements in quality of life.2,9,12 Newer infusion medications, such as lecanemab and donanemab, may delay progression but do not stop or reverse the disease and may cause significant risks like brain swelling and bleeding. These limitations highlight the importance of lifestyle modifications to prevent the onset and progression of AD.3,8,9
Burden of Alzheimer Disease
With increasing life expectancy and age as a primary risk factor, AD represents a growing worldwide burden.3 The Global Burden of Disease classification system ranks AD as the sixth most burdensome condition by disability-adjusted life years (DALYs).2 In 2000, there were an estimated 4.5 million adults diagnosed with AD in the United States; by 2050, this is expected to reach 13.2 million. Approximately 43% of patients with AD require around-the-clock care in nursing homes, placing a significant financial strain on patients, families, and infrastructure.8 The United States is projected to spend more than $600 billion on AD care by 2050.12
The rising prevalence also increases the demand for care partners, including both professionals and family members, to assist with daily activities such as managing medications, finances, transportation, and personal hygiene. Serving as a care partner for someone with AD can be mentally and physically demanding. According to the Alzheimer’s Association, 59% of caregivers report high levels of emotional stress, and 38% report high levels of physical stress.2 Care partners describe greater emotional, physical, and financial hardship caring for individuals with AD compared with other illnesses.2 As care needs increase in the coming years, the implementation of preventative strategies is vital for managing the effects of AD on health care systems and families.
Dietary Components and Mechanisms
Western Diet
The Western diet (WD) is associated with obesity, hypercholesterolemia, cardiovascular (CV) disease, hypertension, and T2DM—all of which are modifiable risk factors for the development and progression of AD.4,9,10,13 It is characterized by high intake of ultra-processed foods and drinks; refined carbohydrates, such as added sugars, white bread, and other processed grain products; unhealthy fats, including saturated fats and refined oils; and excess sodium and red meat.2,10,13 It lacks nuts, legumes, whole grains, fruits, and vegetables, which are sources of essential nutrients, including vitamins, fiber, and antioxidants.3,14
This diet over-emphasizes pro-inflammatory foods that have been linked to pathologic brain changes.2,10,13 For example, consuming significant amounts of high-fructose corn syrup, glucose, and sodium contributes to neuronal loss, brain atrophy, hypertension, and neurodegeneration.3,14 Excessive consumption of red meat increases risk for insulin resistance, atherosclerosis, obesity, systemic inflammation, and the development of T2DM; studies show that this may be among the most important contributors to AD pathology.14 Higher dairy and saturated fatty acid intake is also associated with cognitive decline.6,15 Processing also eliminates healthy fats, antioxidants, and nutrients that provide neuroprotection and reduce the risk for AD progression.3
Mediterranean Diet
The MedDi is extensively studied for cognitive function.3,6 Originating from the region surrounding the Mediterranean Sea, it emphasizes whole, minimally processed produce and healthy fats, abundant fresh fruits and vegetables, legumes, beans, nuts, seeds, whole grains, and extra-virgin olive oil.3,15,16 It also incorporates moderate intake of fatty, non-fried fish, lean white meat, low-fat dairy, potatoes, and eggs.6,16 MedDi foods are rich in omega-3 fatty acids, polyphenols, antioxidants, vitamins C and E, folate, and numerous minerals that are neuroprotective. This diet restricts sugar, fried foods, red or processed meats, refined oils, and high-fat dairy—all of which are linked to modifiable risks for AD.3,6
Growing evidence indicates that the nutrients in the MedDi provide neuroprotection by mitigating AD pathology and modifiable risk factors.7,9,17 Fruits and vegetables contain antioxidants, such as vitamin C, hydroxycinnamic acids, flavonoids, carotenoids, and anthocyanins, which reduce oxidative stress and inflammation. Cold-water ocean fish provide high levels of omega-3 fatty acids and vitamin D, which are anti-inflammatory and neuroprotective.12,14 Vitamin D has also been linked to a reduction in Ab production and improved protein clearance.14 Extra-virgin olive oil lowers LDL-cholesterol, reduces inflammation by inhibiting cyclooxygenase, contains antioxidants and polyphenols, and prevents Ab aggregation.6,12,14 It has been found to reduce the risk for diabetic diseases and protect the CV system through these mechanisms. Legumes supply folate, which reduces levels of homocysteine, a marker for increased AD risk. Overall, the MedDi reduces inflammation, oxidative stress, and risk for T2DM and CV disease via a nutrient-rich style of eating that protects brain health.3,7
MedDi Effects on AD Progression
Substantial evidence supports the role of the MedDi in reducing risk for the development and progression of AD.4,12,14,17 Systematic reviews show a strong association between adherence to the MedDi and reduced cognitive impairment and AD progression.17 Other studies show that the MedDi lowers risk for AD subsequent to MCI6,12,16 and reduces rates of obesity, CV disease, insulin resistance, strokes, and consequently, AD.4,12 Greater adherence has been correlated with improved episodic memory, enhanced global cognition, and defense against AD for up to 3.5 years.6,18 A recent investigation predicted that early implementation of the MedDi could reduce cases of AD by 9 million within the next 4 decades.9
These benefits may relate to specific nutrients as well as overall calorie reduction, and the mechanism warrants further study. Some studies have not demonstrated neuroprotective effects with the MedDi.7 Possible explanations for these findings include inconsistency in levels of specific nutrients consumed by subjects, self-reported data, confounding lifestyle factors, such as exercise and smoking, and irregularities in the scoring systems used to assess cognitive improvement.7,17,18
Conclusion
The lack of pharmacologic interventions to stop or reverse AD progression underscores the need to explore other prevention and treatment approaches.3,5,12 The MedDi has emerged as a promising non-pharmacologic strategy to decrease the risk for cognitive decline in AD.18 Its emphasis on whole, unprocessed foods rich in omega-3 fatty acids, antioxidants, and other neuroprotective nutrients offers a viable option for promoting brain health and delaying the onset of AD.14,16 The MedDi is associated with reduced inflammation and oxidative stress and lower rates of known modifiable risk factors for AD, such as T2DM and CV disease.9,12,14 Although further research is warranted to elucidate the precise mechanisms and validate long-term efficacy in diverse populations,18 the incorporation of MedDi principles into public health and patient care may help reduce the burden of AD and improve quality of life for patients and caregivers.3
This article originally appeared on Clinical Advisor
Muscle synergy-driven ensemble learning framework for individualized stroke gait rehabilitation
Great word salad but is anything in here even approaching a protocol that will deliver EXACT RECOVERY? Since the answer is no; YOU'RE ALL FIRED! The goal of stroke research is survivor recovery! And your mentors and senior researchers are THAT FUCKING INCOMPETENT?
Muscle synergy-driven ensemble learning framework for individualized stroke gait rehabilitation
n
Scientific Reports volume 15, Article number: 44025 (2025) Cite this article
Abstract
This study proposes a novel ensemble machine learning (ML) framework integrating neurophysiological principles from muscle synergy analysis to support clinical decisions in stroke gait rehabilitation. The framework leverages spatial and temporal features of muscle synergies using a hierarchical ensemble model to improve classification performance and interpretability. Muscle synergies were extracted using non-negative matrix factorization from surface EMG recordings of 380 participants, comprising 120 healthy and 260 post-stroke individuals, each contributing one leg. Feature vectors were derived through a bidirectional decomposition process, wherein either the spatial (W) or temporal (H) synergy matrix was fixed to normative patterns obtained from healthy controls. This enabled the extraction of patient-specific deviations in coordination and activation timing, serving as interpretable indicators of stroke-related neuromuscular impairment. Separate classifiers trained on each feature domain were integrated via meta-regression, achieving classification accuracies above 98% across all configurations on the internal test dataset. After training, performance-weighted feature importance values from tree-based models validated the clinical relevance of learned classification criteria. SHAP (SHapley Additive exPlanations) values quantified sample-specific feature contributions on the test dataset, ensuring individual-level interpretability of predictions. The proposed framework bridges stroke-related neuromuscular impairments and clinical insights, laying a foundation for integrating neurophysiologically grounded ML models into rehabilitation.
Introduction
In clinical settings, precise movement assessment facilitates the early diagnosis, treatment planning, and monitoring of neuromuscular impairments. Consequently, movement analysis has become increasingly crucial, particularly in rehabilitation medicine, as it provides a comprehensive understanding of motor function, optimizes performance, and informs individualized rehabilitation strategies1. Over the past few decades, the development of movement analysis has accelerated, particularly with the advent of muscle synergy analysis (MSA), which employs unsupervised machine learning (ML) techniques to elucidate the neurophysiological control of movement2. A key principle of MSA is to uncover the spatial and temporal coordination of the activation of multiple muscles to produce efficient movement, rather than controlling each muscle independently3. Currently, in the fields of neurological4 and musculoskeletal5 diseases, MSA has provided valuable insights into motor control mechanisms by factorizing coordinated activation into spatial and temporal features.
Among various neuromuscular impairments, stroke has been the most frequently investigated using MSA to identify its gait features, due to its high incidence rate6. Notably, stroke is the second leading cause of death worldwide7 and causes persistent post-survival motor impairments that significantly affect the quality of life8. Many studies have elucidated specific patterns of gait dysfunction in stroke patients, such as the phenomenon of synergy merging, in which multiple muscle synergies combine into a single, less efficient, and specific synergy during gait9,10. Recovery from these pathological gait patterns is therefore considered a critical target in stroke rehabilitation to restore more normalized gait.
Several prior studies have applied muscle synergy features, particularly temporal coefficients, in combination with tree-based ML models to assess motor impairment and predict rehabilitation outcomes in stroke patients. For example, Yang et al. demonstrated the utility of temporal muscle synergy features by applying random forest classifiers to characterize impairment severity during sit-to-stand tasks11 and to estimate the effects of short-term rehabilitation12. An et al. further extended this approach to kinetic assessments using handrail force data in severely affected patients13. While these studies focused on temporal features to assess functional states, our study integrates both gait-specific temporal and spatial features, placing particular emphasis on model-level interpretability for pre-rehabilitation decision-making.
By utilizing MSA along with knowledge to understand factorized features, clinicians and practitioners can develop targeted rehabilitation protocols, optimize training strategies, and refine therapeutic interventions to improve gait performance in stroke patients. However, its clinical adoption remains limited due to ambiguities in interpreting and prioritizing synergy features, which often requires substantial practitioner expertise and introduces variability in application14,15,16. Therefore, in this study, we propose that an ensemble ML framework that highlights clinically relevant synergies may facilitate effective translation into practice. To the best of our knowledge, despite the introduction of various ML models and advancements in computer-aided diagnostic systems designed to reduce subjectivity and improve the consistency in assessments using electromyography (EMG)17,18, the application of MSA for therapeutic decision-making within an ML framework remains extremely rare. In particular, our approach introduces a bidirectional decomposition strategy anchored to normative synergy templates and incorporates SHAP (SHapley Additive exPlanations)-based individualization to enhance interpretability and clinical relevance.
In addition to their use in clinical diagnostics, EMG signals have been widely applied in robotics to train ML algorithms for controlling prosthetic arms and legs. This process typically involves a series of preprocessing steps such as signal filtering, normalization, and feature extraction in both the time and frequency domains. Among these steps, segmentation window size is critical, as it influences both temporal resolution and feature reliability. Shorter windows enhance temporal precision but increase variability, while longer windows stabilize signals but may miss transient events19. Commonly extracted features include root mean square, mean absolute value, median frequency, and wavelet coefficients. These features are then used as inputs for various ML models, including support vector machines, random forests, and deep learning architectures, to predict motor intentions and classify movement patterns. However, these studies have primarily focused on improving the model performance, often overlooking the interpretability of the contributing features or the physiological relevance of the learned representations20,21.
To address these limitations, this study proposed a framework aimed at enhancing the clinical interpretability of EMG data by extracting physiologically meaningful features using MSA. These features were subsequently used to train an ensemble ML model. Feature importance metrics and SHAP analysis were applied to identify synergy components that most influenced the model’s output, enabling individualized prioritization of muscle synergies, which refers to selecting the most relevant features for each patient based on SHAP-derived importance values. Furthermore, a voting-based classification approach was adopted by integrating four distinct tree-based ML models to ensure performance robustness across varying data characteristics. To support reliable interpretation, model-specific weights were assigned based on each model’s predictive performance when aggregating SHAP values and feature importance.
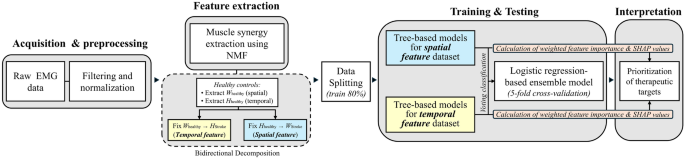
Notably, the spatial and temporal features derived from MSA exhibit distinct structural and statistical characteristics. Separate voting classification models were developed for each feature type and subsequently integrated into a unified ensemble model (Fig. 1). This approach provides a unified framework for decision making by consolidating predictions from both feature sets into a single model.
Accordingly, the proposed framework may offer several stage-specific advantages along the analytical pipeline. First, the use of non-negative matrix factorization (NMF) to extract muscle co-activation patterns (spatial features) and activation timings (temporal features) allows clinicians to identify which muscle groups and gait phases may be most influential in each patient. Second, these quantified features can be translated into a preliminary prioritization scheme, guiding therapists toward muscle synergies that the model indicates as clinically significant. Third, because the framework summarizes its outputs using familiar visualization tools such as feature importance scores and SHAP plots, it supports clinical interpretation without requiring expertise in ML, potentially improving usability in practice.
Overview of the muscle synergy-driven ML framework for stroke gait rehabilitation: from EMG to SHAP-informed therapeutic decision-making.
Results
Extracted muscle synergy
Four muscle synergies were extracted from all participants based on the criterion that collectively accounted for over 90% of the EMG variance in healthy controls (variance accounted for, VAF = 0.95 ± 0.03). This number aligns with previously reported synergy structures identified during human locomotion22,23.
Numerous studies on MSA have demonstrated that NMF serves as a robust method for uncovering modular motor control strategies, as it enforces physiological non-negativity constraints and aligns closely with the structure of real-world muscle activation patterns22,23. By grouping co-activated muscles into low-dimensional modules, NMF captures the functional coordination among individual muscles, thereby enhancing the interpretability of complex motor behaviors. In this study, each extracted synergy was explicitly linked to its physiological role based on dominant muscle contributions and their established biomechanical functions during gait.
As summarized in Table1, Synergy 1 (S1) predominantly involves the hamstring muscles, including the biceps femoris (BFM) and semimembranosus (SEM), functioning primarily for deceleration and stabilization of the leg during the terminal swing and early stance phases. Synergy 2 (S2), characterized by strong activation of Rectus Femoris (RFM) and Adductor Magnus (ADD), contributes to knee extension and hip adduction, playing a key role in load acceptance and medial-lateral control during the early stance phase. Synergy 3 (S3), predominantly involving the Gastrocnemius (GCM), is critical for forward propulsion and push-off during terminal stance and pre-swing phases. Finally, Synergy 4 (S4), involving Gluteus Medius (GLU), Vastus Medialis (VMM), and Tibialis Anterior (TIB), supports pelvic stability and stance phase control, particularly during mid-stance24,25..
As shown in Fig. 2, visual comparison revealed that in patients with stroke, the muscles contributing to individual synergies (spatial features) were generally more strongly activated than in normal controls, and the timing of synergy activation (temporal features) appeared ambiguous and delayed.
Moreover, to assess the impact of stroke on synergy organization, we applied a bidirectional decomposition strategy. Extracting the spatial synergy features of stroke patients with fixed temporal features from healthy controls revealed a significant decrease in VAF (0.19 ± 0.16), indicating severely disrupted muscle coordination post-stroke. Conversely, extracting temporal synergy features with fixed spatial patterns resulted in a relatively higher VAF (0.70 ± 0.10). These findings support the hypothesis that spatial features (muscle coordination) are more substantially affected by stroke compared to temporal features (activation timing), consistent with previous clinical observations of altered neuromuscular recruitment patterns in post-stroke gait25,26.
By clearly relating NMF-derived synergy structures to established biomechanical functions and clinical phenomena, our proposed framework significantly enhances interpretability and facilitates clinical decision-making for targeted and individualized gait rehabilitation.
More at link.