But is it better than following the Margaret Yekutiel book about this in 2001, 'Sensory Re-Education of the Hand After Stroke'?
A new electromechanical trainer for sensorimotor rehabilitation of paralysed fingers: A case series in chronic and acute stroke patients
Journal of NeuroEngineering and Rehabilitation volume 5, Article number: 21 (2008)
Abstract
Background
The functional outcome after stroke is improved by more intensive or sustained therapy. When the affected hand has no functional movement, therapy is mainly passive movements. A novel device for repeating controlled passive movements of paralysed fingers has been developed, which will allow therapists to concentrate on more complicated tasks. A powered cam shaft moves the four fingers in a physiological range of movement.
Methods
After refining the training protocol in 2 chronic patients, 8 sub-acute stroke patients were randomised to receive additional therapy with the Finger Trainer for 20 min every work day for four weeks, or the same duration of bimanual group therapy, in addition to their usual rehabilitation.
Results
In the chronic patients, there was a sustained reduction in finger and wrist spasticity, but there was no improvement in active movements. In the subacute patients, mean distal Fugl-Meyer score (0–30) increased in the control group from 1.25 to 2.75 (ns) and 0.75 to 6.75 in the treatment group (p < .05). Median Modified Ashworth score increased 0/5 to 2/5 in the control group, but not in the treatment group, 0 to 0. Only one patient, in the treatment group, regained function of the affected hand. No side effects occurred.
Conclusion
Treatment with the Finger Trainer was well tolerated in sub-acute & chronic stroke patients, whose abnormal muscle tone improved. In sub-acute stroke patients, the Finger Trainer group showed small improvements in active movement and avoided the increase in tone seen in the control group. This series was too small to demonstrate any effect on functional outcome however.
Introduction
The annual stroke incidence is approximately 180 patients per 100,000 inhabitants in the industrialized world. About 30% of the surviving patients suffer from a severe upper limb paresis with a non functional hand. The prognosis for regaining meaningful hand activity six months after stroke onset is poor [1]: this may partly be because current rehabilitation practice puts more emphasis on the compensatory use of the non-affected upper extremity [2].
Powered machines which can allow prolonged repetition of a controlled movement are a promising way of increasing the intensity of rehabilitation after stroke. Several devices, to treat wrist, elbow & shoulder movements, have been developed since the pioneering MIT-Manus in the early 1990s [3]. Randomized controlled trials show a convincing beneficial effect of robot-assisted upper limb treatment on the impairment of severely affected stroke patients [4–9].
There are fewer clinical reports of machine-assisted movement of paralysed fingers. The Rutgers Hand Masters I and II use pistons mounted inside the palm to move the fingers, with virtual reality to improve motivation. Chronic stroke patients improved range of motion, motor control and speed of the paretic fingers over several weeks of training, and the benefits were retained at follow-up [10, 11].
With the Howard Hand Robot, pistons assist with patient initiated grasping and releasing movements around virtual or real objects. In moderately affected chronic stroke subjects, upper limb motor functions improved, and functional MRI revealed increased sensorimotor cortex activation during the grasping task which was not seen during a non-practiced task, supination/pronation [12].
Fischer et al assisted the finger extension of mildly affected stroke patients with the help of a powered orthosis. Following six weeks of training in reach-to-grasp of virtual and actual objects, patients' active motor performance had shown a moderate improvement [13].
The treatment of the plegic fingers after stroke is pertinent given their large cortical representation, the presumed competition between proximal and distal limb segments for plastic brain territory [14], and recent results from the MIT-group promoting earlier active treatment of distal limb [15]. Further, paresis-related immobilization seems to contribute to the development of long-term disabling finger flexor spasticity [16].
We have designed an electromechanical Finger Trainer to move individual fingers in a physiological range of movement. This article describes the device and reports its use in a small number of chronic and acute stroke patients with completely paralysed hands.
Device
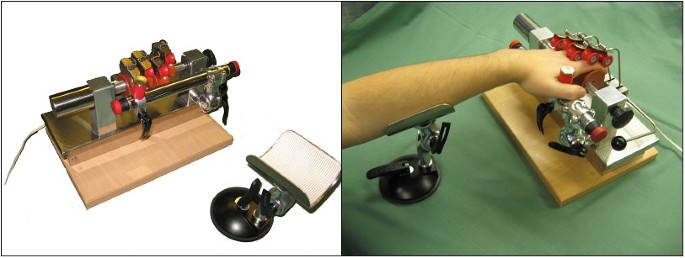
The Finger Trainer, Reha-Digit, (figure 1) consists of four, mutually independent plastic rolls, each fixed eccentrically to the powered axle of the device, forming a cam-shaft. Each finger-roll can be repositioned & secured by turning a knob on the main axle, on the other end from the motor, to fit the size & range of movement of each individual finger.
The surface of each finger roll is concave, forming a gutter to maximise the contact area between finger & roll. Two smaller locking rollers, also concave, hold each finger against the larger finger roll. Each pair of locking rollers moves orthogonally to the axis of the finger roll, and an elastic spring pulls each pair of locking rollers towards the finger roller. These can be lifted out of the way when first positioning the hand & fingers in the device.
A spacing bar, parallel to the drive axle, holds the hand in the optimal position: a thumb stop may be used to provide additional stability. This can be moved to either side, to accommodate either the left or right hand. There are emergency-stop switches at each end of the spacing bar. The forearm can be stabilised at the correct angle & height on a gutter support.
A 24 V DC motor rotates the drive axle up to 30 times a minute through a clutch mechanism, which allows the axle to stop rotating if the hand goes into a powerful spasm. A vibration engine, situated under the base plate, provides small amplitude (2 mm) stimulation at a frequency which can be set between 0 to 30 Hz, by turning a knob. The device's weight is 7 kg, and its dimensions are 35 cm × 24 cm × 22 cm.
Treatment
The patient sat comfortably on a chair with a backrest, with the device on a height-adjustable table in front of him. A therapist positioned the forearm on the arm support, placed the patients' four fingers II – V onto the cam shaft, and placed the thumb behind the spacing-bar or under the thumb-stop. The patient should not report any pain. In case of severe finger flexor spasticity, the therapist manually reduced the muscle tone before putting the hand in the device, and ultrasound contact gel could be applied to the fingers to diminish the friction between fingers and finger-rolls.
Initially, the rotation speed of the cam shaft and the vibration frequency were set at 20 rotations per minute and 20 Hz. After three minutes the treatment was interrupted in order to modify the treatment conditions regarding positioning, rotation speed and vibration frequency. The patient practised a total of 15 min with the device. The patients were instructed to concentrate on the movement of the paretic fingers and, if possible, to imagine that they themselves performed the finger movements.
To avoid saturation of the Meissner organs by continuous tactile dynamic stimulation of the finger tips by the revolving rolls, strips with different surface texture were attached to the inner surface of the concave roll of the index finger, and the patients were asked to discriminate between them. Patients with arthritis of the finger joints, soft tissue pain or hand swelling were excluded.
No comments:
Post a Comment