Yeah, this is for peripheral nerves, but doesn't your competent? doctor have enough functioning brain cells to test this out for stroke recovery?
Oriented Graphene Oxide Scaffold Promotes Nerve Regeneration in vitro and in vivo
Authors Zhou X , Tang A, Xiong C , Zhang G , Huang L, Xu F
Received 24 October 2023
Accepted for publication 14 February 2024
Published 13 March 2024 Volume 2024:19 Pages 2573—2589
DOI https://doi.org/10.2147/IJN.S439656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lijie Grace Zhang
Xu Zhou,1,2,* Aolin Tang,2,3,* Chengjie Xiong,2,* Guoquan Zhang,2 Liangliang Huang,1,2 Feng Xu1,2
1The First School of Clinical Medicine, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 2Department of Orthopaedics, General Hospital of Central Theater Command, Wuhan, 430070, People’s Republic of China; 3Department of Orthopaedics, Minda Hospital of Hubei Minzu University, Enshi, 445000, People’s Republic of China
*These authors contributed equally to this work
Correspondence:
Liangliang Huang; Feng Xu, Department of Orthopaedics, General Hospital
of Central Theater Command, Wuhan, 430070, People’s Republic of China,
Email hll666789@163.com; fengxu1969@163.com
Background:
Treating peripheral nerve injuries (PNI) with defects remains
challenging in clinical practice. The commercial conduits have shown
suboptimal nerve regeneration and functional recovery due to their basic
tubular design without electroactive and oriented topographical cues.
Purpose:
To develop a new scaffold with oriented microstructure and
electroactive Graphene oxide (GO) and investigate its’ therapeutic
effect on nerve regeneration in vitro and in vivo.
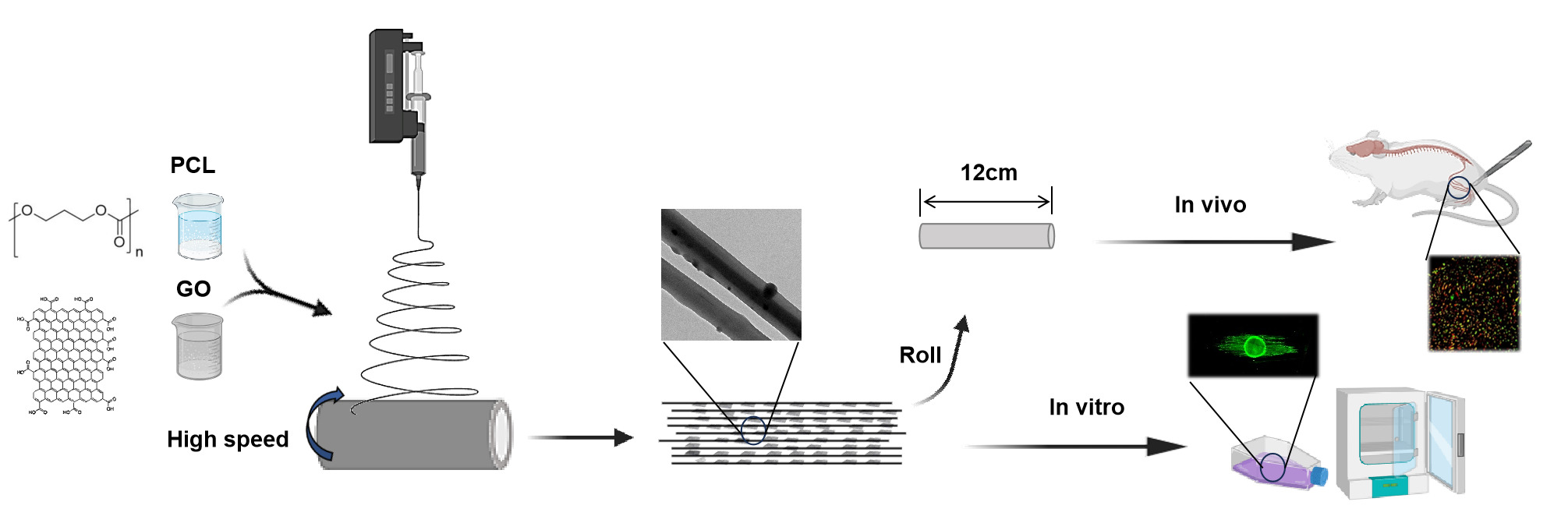
Methods:
This study employed a straightforward approach to co-spin PCL and GO,
yielding an oriented hybrid nanofibrous scaffold known as the O-GO/PCL
scaffold. The physical and chemical properties of nanofibrous scaffold
were tested by scanning electron microscopy (SEM), transmission electron
microscope (TEM), tensile test and so on. Primary Schwann cells (SCs)
and dorsal root ganglia (DRG) were used to investigate the impact of the
newly developed scaffolds on the biological behavior of neural cells in
vitro. Transcriptome sequencing (mRNA-seq) was employed to probe the
underlying mechanisms of the synergistic effect of electroactive GO and
longitudinal topographic guidance on nerve regeneration. Furthermore,
the developed O-GO/PCL scaffold was utilized to bridge a 10-mm sciatic
nerve defect in rat, aiming to investigate its therapeutic potential for
peripheral nerve regeneration in vivo.
Results and discussion:
The SEM and TEM revealed that the newly developed O-GO/PCL scaffold
showed longitudinally oriented microstructure and GO particles were
homogenously and uniformly distributed inside the nanofibers. Primary
SCs were utilized to assess the biocompatibility of the GO-based
scaffold, revealing that negligible cytotoxicity when GO concentration
does not exceed 0.5%. In vitro analysis of nerve regeneration
demonstrated that axons in the O-GO/PCL group exhibited an average
length of 1054.88 ± 161.32 μm, significant longer than those in the
other groups (P < 0.05). Moreover, mRNA sequencing results
suggested that the O-GO/PCL scaffold could enhance nerve regeneration by
upregulating genes associated with neural regeneration, encompassing
ion transport, axon guidance and cell–cell interactions. Most
importantly, we employed the O-GO/PCL scaffold to repair a 10-mm sciatic
nerve defect in rat, resulting in augmented nerve regeneration,
myelination, and functional recovery.
Conclusion: The O-GO/PCL scaffold with oriented microstructure and electroactive GO represents a promising heral nerve reconstruction.
Keywords: nanofibers, topographical guidance, electroactive, graphene oxide, nerve regeneration
Graphical Abstract:
Introduction
Peripheral nerve injury represents a prevalent global health issue, impacting over 5 million individuals worldwide.1,2 Despite significant advancements in microsurgical techniques in recent years, effectively addressing nerve defects remains a formidable challenge. While autografts have stood as the gold standard for nerve defect repair, they are plagued by issues like donor nerve scarcity, secondary trauma, and neuroma formation.3 In recent years, artificial nerve conduits, including NeuroTube, NeuraGen, NeuroMatrix, and others, have been developed as an alternative approach.4 However, these commercial conduits have shown suboptimal nerve regeneration and functional recovery due to their basic tubular design that offers only general guidance.5,6 Consequently, there is a pressing need for innovative solutions with enhanced effectiveness for nerve repair in clinical scenarios.
It is now understood that following peripheral nervous system (PNS) injuries, the distal nerve stump undergoes Wallerian degeneration and forms aligned Büngner bands, facilitating and promoting axon growth. Inspired by the organized structures of the PNS, longitudinal microstructures such as aligned fibers, microgrooves, and microchannels have been introduced into nerve guidance scaffolds (NGSs) using various methods like electrospinning, phase separation, microstereolithography, template thermo-crosslinking, and mold freeze-drying.7–11 Electrospinning offers numerous advantages, including ease of use, customizable scaffold structure, adjustable fiber diameter, and suitability for various materials. State-of-the-art techniques have enabled the production of highly aligned fibers that mimic tissue structures, thereby improving regeneration.12,13 For instance, Wang et al demonstrated that highly aligned electrospun poly-L-lactic acid (PLLA) fibers with appropriate diameters encouraged neurite extension and Schwann cell (SC) migration.14 Besides, Zhang et al reported that aligned nanofibers offered optimal topographical cues for guiding cell and neurite growth in specific orientations.15
Beyond topographical cues defined by the scaffold’s microstructure, the significance of electroactive functional materials has gained prominence.16–18 Among these conductive materials, graphene oxide (GO) stands out due to its exceptional electrical conductivity, biocompatibility, and favorable interactions with cell interfaces. The negative carboxylate groups on GO enhance colloidal stability and hydrophilicity, making it suitable for surface attachment, proliferation, and differentiation of nerve cells.19 Li et al demonstrated that GO significantly stimulates neural cell proliferation and differentiation.20 Importantly, SCs exhibited improved adhesion, proliferation, and neurotrophic factor secretion on GO-based substrates. GO has also been shown to enhance neuro-specific gene expression, neurite outgrowth, and sprouting in neuronal-like cells.21
Recently, a significant amount of effort has been taken in developing nerve scaffolds with oriented microstructures and electroactive GO to promote nerve regeneration. Most of these strategies have successfully coated GO onto 2D surfaces. However, the coated GO is easy to detach from the 2D surfaces. Thus, it is challenging to translate them into 3D implantable scaffolds for clinical use.15,22 Furthermore, most of these studies have just investigated the effect of oriented microstructures and electroactive GO on nerve regeneration using cell lines (such as PC12 and RSC96) in vitro. Notably, the cell lines are tumor-like cells, which show different biological characteristics compared with primary cells and in vivo situations. Moreover, the mechanisms underlying the synergistic impact of electroactive GO and oriented topographic guidance on nerve regeneration remains largely unexplored. Thus, for potential clinical applications, it is imperative to adopt a more straightforward approach to fabricate nerve scaffolds with electroactive GO and oriented microstructures topography, assess the therapeutic effect of the scaffolds using primary cell cultures and in vivo animal models, and comprehend the underlying mechanisms.
In the present study, we employed a straightforward technique to co-spin polycaprolactone (PCL) and GO, resulting in an oriented GO/PCL hybrid nanofibrous scaffold termed the O-GO/PCL scaffold. The physical and chemical properties of nanofibrous scaffold were tested by scanning electron microscopy (SEM), transmission electron microscope (TEM), tensile properties, 4-point probe method, Fourier transform infrared (FTIR), and contact angle analysis. The impact of these developed scaffolds on the biological properties and behavior of neural cells was examined through co-culturing with primary SCs and dorsal root ganglia (DRG) in vitro. Transcriptome sequencing (mRNA-seq) was employed to probe the underlying mechanisms of the synergistic effect of electroactive GO and longitudinal topographic guidance on nerve regeneration. Furthermore, the developed O-GO/PCL NGS was utilized to bridge a 10 mm sciatic nerve defect in rats, aiming to investigate its therapeutic potential for peripheral nerve regeneration in vivo.
No comments:
Post a Comment