You'll have to ask your competent? doctor why the hell edaravone is approved in Japan since 2001 but not the US.
Has your stroke hospital done anything with edaravone in the last decade?
edaravone (12 posts to November 2011)
Update on Antioxidant Therapy with Edaravone: Expanding Applications in Neurodegenerative Diseases
1
Department of Neurology, Okayama
University Graduate School of Medicine, Dentistry and Pharmaceutical
Sciences, 2-5-1 Shikata-cho, Okayama 700-8558, Japan
2
Department of Neurology, National Center of Neurology and Psychiatry, Tokyo 187-8551, Japan
*
Author to whom correspondence should be addressed.
Int. J. Mol. Sci. 2024, 25(5), 2945; https://doi.org/10.3390/ijms25052945
Submission received: 26 January 2024
/
Revised: 19 February 2024
/
Accepted: 29 February 2024
/
Published: 3 March 2024
(This article belongs to the Special Issue Antioxidants in Health and Diseases)
Abstract
The brain is susceptible to oxidative stress, which
is associated with various neurological diseases. Edaravone (MCI-186,
3-methyl-1 pheny-2-pyrazolin-5-one), a free radical scavenger, has
promising effects by quenching hydroxyl radicals (∙OH) and inhibiting
both ∙OH-dependent and ∙OH-independent lipid peroxidation. Edaravone was
initially developed in Japan as a neuroprotective agent for acute
cerebral infarction and was later applied clinically to treat
amyotrophic lateral sclerosis (ALS), a neurodegenerative disease. There
is accumulating evidence for the therapeutic effects of edaravone in a
wide range of diseases related to oxidative stress, including ischemic
stroke, ALS, Alzheimer’s disease, and placental ischemia. These
neuroprotective effects have expanded the potential applications of
edaravone. Data from experimental animal models support its safety for
long-term use, implying broader applications in various
neurodegenerative diseases. In this review, we explain the unique
characteristics of edaravone, summarize recent findings for specific
diseases, and discuss its prospects for future therapeutic applications.
1. Introduction
The
brain, which is rich in lipids and exhibits high oxygen consumption, is
susceptible to damage via oxidative stress. Briefly, oxidative stress
is caused by an imbalance between the production and accumulation of
reactive oxygen species (ROS) in cells and tissues and the ability of a
biological system to detoxify these reactive products [1]. ROS contribute to several physiological processes (e.g., cell signaling) [2]
and are generated as byproducts of oxygen metabolism under normal
conditions. Nevertheless, environmental stressors and xenobiotics can
contribute to a significant increase in ROS production, resulting in
cellular and tissue damage. Oxidative stress has been implicated in
neurodegenerative diseases, including amyotrophic lateral sclerosis
(ALS), Parkinson’s disease, Alzheimer’s disease (AD), Huntington’s
disease, depression, and multiple sclerosis. Furthermore, it plays an
important role in the pathogenesis of acute ischemic stroke [3,4]. Free radical formation and subsequent oxidative damage may be a factor in stroke severity [5].
Therefore, several antioxidants with demonstrated or predicted
beneficial effects against oxidative stress and stroke have recently
been reported [6,7,8,9].
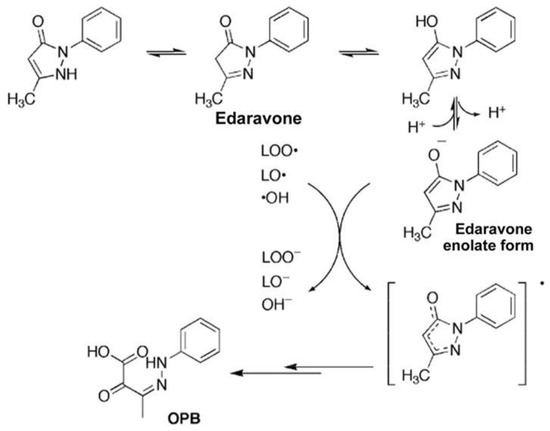
Edaravone (MCI-186, 3-methyl-1 pheny-2-pyrazolin-5-one) was first established in Japan as a free radical scavenger (Figure 1).
It was initially approved for the treatment of acute ischemic stroke in
Japan, where it is manufactured under the brand name Radicut. It has
been used in clinical practice for the treatment of acute cerebral
ischemia and ALS owing to its antioxidative and anti-inflammatory
effects. Edaravone has also been approved for use in Japan, South Korea,
and the United States for the treatment of ALS. It has been approved in
the United States under the brand name Radicava by the U.S. Food and
Drug Administration (FDA).
Figure 1.
Reaction mechanism of edaravone with free radicals (revised from Nakagawa et al. 2006 [10]).
The enolate form of edaravone interacts with both peroxyl radicals
(LOO·) and hydroxy radicals (·OH) to form stable oxidation products
(2-oxo-3-(phenylhydrazono)-butanoic acid; OPB).
In this study, using PubMed, we searched the
literature for studies related to edaravone for a comprehensive review
of its development and applications to various diseases. We introduce
the unique characteristics of edaravone, summarize recent findings for
various diseases (Table 1 and Table 2), and discuss prospects for future therapeutic applications.

No comments:
Post a Comment