Ask your doctor if anything here is going to get you closer to 100% recovery. If your doctor doesn't even know about this; YOU DON'T HAVE A FUNCTIONING STROKE DOCTOR! I don't know what you have but that person is not keeping up-to-date on stroke research!
NSF DARE—Transforming modeling in neurorehabilitation: Four threads for catalyzing progress
Journal of NeuroEngineering and Rehabilitation volume 21, Article number: 46 (2024)
Abstract
We present an overview of the Conference on Transformative Opportunities for Modeling in Neurorehabilitation held in March 2023. It was supported by the Disability and Rehabilitation Engineering (DARE) program from the National Science Foundation’s Engineering Biology and Health Cluster. The conference brought together experts and trainees from around the world to discuss critical questions, challenges, and opportunities at the intersection of computational modeling and neurorehabilitation to understand, optimize, and improve clinical translation of neurorehabilitation. We organized the conference around four key, relevant, and promising Focus Areas for modeling: Adaptation & Plasticity, Personalization, Human-Device Interactions, and Modeling ‘In-the-Wild’. We identified four common threads across the Focus Areas that, if addressed, can catalyze progress in the short, medium, and long terms. These were: (i) the need to capture and curate appropriate and useful data necessary to develop, validate, and deploy useful computational models (ii) the need to create multi-scale models that span the personalization spectrum from individuals to populations, and from cellular to behavioral levels (iii) the need for algorithms that extract as much information from available data, while requiring as little data as possible from each client (iv) the insistence on leveraging readily available sensors and data systems to push model-driven treatments from the lab, and into the clinic, home, workplace, and community. The conference archive can be found at (dare2023.usc.edu). These topics are also extended by three perspective papers prepared by trainees and junior faculty, clinician researchers, and federal funding agency representatives who attended the conference.
Introduction
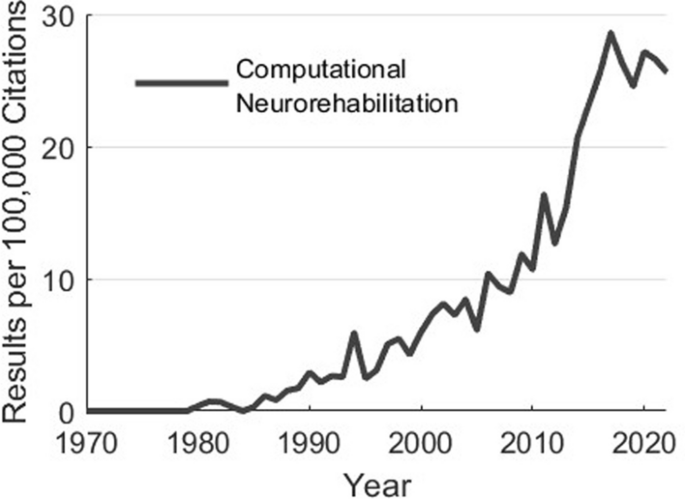
Why do we create computational models? Simply put, to help us move from costly and inefficient trial-and-error empiricism towards mechanistic, hypothesis-driven, and evidence-based systematic processes to develop clinical treatments and products [1, 2]. Along these lines, computational modeling has had profound impacts on our scientific understanding. For example, computational models of sensorimotor control and plasticity underlie the design and application of neuromodulatory approaches to enhance motor function during development, aging, and following neurological injury or disease [3,4,5,6,7,8,9,10,11]. Additionally, computational musculoskeletal models have been used to inform treatment decisions in orthopedics and sports medicine. Computational models describing the trajectories of development, disability, and recovery have the potential to help prioritize and focus treatment. Moreover, models of musculoskeletal dynamics and neural control are regularly used by researchers to design and implement control strategies of assistive technologies [12,13,14,15,16,17,18]. The impacts of computational modeling are only set to increase in the coming decades: the emergence of multimodal remote sensors, machine learning, and multiscale datasets (from genomics to behavior) will enable a future in which personalized neurorehabilitation that adapts throughout the course of treatment becomes the norm. In fact, the interest in these areas continues to accelerate (Fig. 1).
As interest and potential impact in these areas accelerates, we convened the NSF DARE Conference: Transformative Opportunities for Modeling in Neurorehabilitation that brought together experts and trainees to discuss critical questions, challenges, and opportunities at the intersection of computational modeling and neurorehabilitation. We identified four Focus Areas prior to the conference through discussions amongst the PI team, Advisory Board, and federal funding representatives. These areas were identified as areas of high growth and potential impact for computational modeling in rehabilitation research (Table 1). They represent—in our opinion—pressing challenges and timely opportunities within neurorehabilitation where advances in science, computational methods, and implementation can converge for actionable change. These areas also exemplify the potential for innovation and impact when merging multiple modeling methods (e.g., machine learning and physics-based models) with technology (e.g., exoskeletons or wearable sensors) to support scientific understanding, target neurorehabilitation outcomes, and improve quality of life. These areas are highly synergistic with NSF’s Disability and Rehabilitation Engineering Program and NIH’s 2021 Research Plan on Rehabilitation.
In this paper we summarize and comment on the perspective and insights from the expert community assembled to help establish a foundation by which computational modeling can create the scientific directions, theories, and actionable platforms to improve the efficacy and personalization of neurorehabilitation. This is based upon discussions with participants during the meeting in general, and among the co-authors during the writing of this paper. Additional concluding remarks are included in the three other companion papers that reflect key take-away points from other conference constituents (e.g., trainees, federal funding representatives, and clinician scientists). Mirroring the structure of the meeting, we now visit and comment on each focus area. It is important to note that the main conclusions are summaries drawn by the authors. As such, they represent the viewpoints of the authors, and not a consensus process of conference attendees. The companion papers further clarify this distinction where each set of authors provides their point of view and provide further conclusions and key take-aways from other constituent groups who participated in the conference (e.g., trainees, clinician scientists, and federal funding representatives). Those additional three papers in this same issue are titled, respectively:
-
NSF DARE—Transforming Modeling in Neurorehabilitation: A Patient-in-the-Loop Framework
-
NSF DARE—Transforming Modeling in Neurorehabilitation: Clinical Insights for Personalized Rehabilitation
-
and
-
NSF DARE—Transforming Modeling in Neurorehabilitation: Perspectives and Opportunities from US Funding Agencies
The field of computational neurorehabilitation has grown tremendously over the preceding decades. Usage of the terms “computational” and “neurorehabilitation” in articles indexed by Pubmed. Data generated by Pubmed by Year (https://esperr.github.io/pubmed-by-year/)
Modeling adaptation and plasticity
Brief background
Adaptation and plasticity are central to our nervous system’s ability to acquire new abilities, adapt them to changing situations, and recover function after injury. The role of synaptic plasticity in sensorimotor learning and adaptation is the subject of much work described in several reviews [19,20,21,22]. Here, our main interest is the role of plasticity in the recovery of function after injury for neurorehabilitation. For example, after stroke, neurons re-wire connections both immediately surrounding the injury and across distant brain areas [23,24,25,26,27,28], and these changes in connectivity are correlated with improvements in function with and without rehabilitation [27, 28]. Critically, adaptation and plasticity are not guaranteed to be fast, well-guided, or beneficial (i.e., adaptive plasticity). Plasticity can also be maladaptive [29, 30], a neurorehabilitation analog to focal dystonias [31, 32].
A key goal of neurorehabilitation therapies is to promote plasticity mechanisms that improve function while also mitigating maladaptive changes to neural circuits. Many therapeutic approaches have been proposed and attempted to achieve this goal—with varying degrees of success—ranging from targeted behavioral training to implantable devices that stimulate neural circuits.
Computational modeling of adaptation and plasticity can provide paths to maximize the impact of neurorehabilitation therapies [3, 33]. The plasticity and adaptation that occurs after injury span many levels of the nervous system, from cellular processes (e.g., changes in ion channel expression) [34], to network interactions (e.g., changes in synaptic connections) [27], to behavioral changes (e.g., compensatory strategies) [35]. As a result, a wide range of modeling methods have been used to describe plasticity at different levels [36]. In lieu of an exhaustive survey of existing models, we highlight some useful categories of model types. For a particular phenomenon, such as synaptic plasticity, models may focus on different levels of abstraction. For example, phenomenological models like Hebb’s rule describe input-output relationships between the rate/timing of neural activity and connection changes, describing the computational principle without directly modeling the biological implementation [37]. Biophysical models of spike-timing dependent plasticity, in contrast, describe the physiological changes within neurons that give rise to synaptic changes [38]. Data-driven models (i.e., machine learning) have also been employed for modeling a variety of plasticity phenomena (e.g., [39]).
A variety of model types, spanning different levels of the nervous system, have been used to describe how the nervous system will respond to an intervention to inform rehabilitation therapies [3, 40]. Mechanistic models of how behavior evolves as we adapt to altered dynamics like a split-belt treadmill, for example, informed training interventions to improve gait post-stroke [41]. Phenomenological Hebbian plasticity models informed stimulation protocols to increase the functional connections among regions in the brain [11, 42, 43], and from the brain to muscles or spinal circuits [44,45,46,47]. Models describing nervous system changes over time are also valuable for predicting outcomes and to guide clinical decision-making. Many examples of these models rely on data-driven discovery from large datasets. For example, the increasing prevalence of neural imaging technologies in clinical practice have led to large datasets to develop algorithms that predict functional recovery after stroke [48]. Machine learning approaches have also been used to assess whether devices like non-invasive brain-computer interfaces will be effective [49, 50] and optimal parameters for therapies like deep brain stimulation [51].
These examples highlight the diversity of plasticity models and applications in neurorehabilitation. As with any other computational modeling effort [2], decisions must be made about the level of abstraction and detail. The challenge of these decisions is acutely clear in the realm of adaptation and plasticity, where mechanisms span spatial scales from synapses to behavior, and timescales from milliseconds to months [20]. Many existing models used for neurorehabilitation focus on a single scale (e.g., describing behavioral changes). Models that bridge neurological mechanisms of plasticity to behavior will likely be needed to improve the precision of neurorehabilitation therapies. Such models will require cross-disciplinary collaboration to develop and validate. Similarly, most existing models focus on describing a particular time-point, such as functional recovery after a certain time with a particular, static therapy. The many time-scales of adaptation and plasticity present challenges for modeling overall trajectories, including the impact of interventions and changes in treatment over time [52].
Extending models of plasticity to span spatial and temporal scales could open new ways to harness the power of computational methods in neurorehabilitation. The dynamic nature of the nervous system creates a variety of challenges for building therapies. Assuring an assistive device provides meaningful functionality for extended periods of time requires characterizing plasticity that may occur in response to the device and developing devices that can adapt accordingly [53]. Similar considerations are needed for therapies where protocols may need to adapt over time as abilities change [54]—a form of meta-adaptation that mirrors meta-plasticity (i.e., ‘plasticity of plasticity’ [36]). Achieving the goal of smart, personalized, and adaptive neurorehabilitation therapies will require models that can capture the dynamics of plasticity processes as well as human-device interactions that will influence those dynamics. This will require new approaches to bridge across models that predict how the nervous system will change in response to a given intervention and those to describe how interventions inpact the trajectory of changes in the nervous system and changes in behavior over time.
Commentary
The DARE workshop highlighted many fundamental challenges and opportunities in modeling adaptation and plasticity for rehabilitation applications. Multiple presentations (see Appendix for speaker summaries) speak to the promise of using computational models to disentangle diverse learning mechanisms used by the nervous system (e.g., Roth, Mariscal). Mechanistic models such as those used by Roth shed light on the neurophysiological underpinnings of disorders. Their findings, for instance, suggest that Parkinson’s disease can lead to deficits in a single learning mechanism while leaving others intact. Similarly, data-driven methods to identify components of learning used by Mariscal allowed them to characterize how learning generalized to new contexts more precisely than past studies. A critical next step missing from most workshop submissions is using these model-derived insights to directly guide clinical therapies. Future work towards these efforts will have to contend with challenges closely related to those faced in personalization efforts (see below). For example, do models and their parameters need to be estimated on populations of people or individuals? Models may also require updating over time as learning proceeds, closely mirroring challenges faced in human-device interactions.
Multiple presentations (e.g., Liew, Orsborn, Hight, Schwock) aimed to characterize plasticity that occurs as the result of therapies and interventions or injuries. Schwock’s work highlights the potential benefits of computational models to quantify changes between regions of the nervous system when they are embedded within a large network (see also [55]). This work highlights the challenge of identifying the most useful measures of nervous system plasticity, since nearly all metrics will be approximations. Data-driven studies probing how physiological measurements relate to clinical outcomes will likely be critical to identify the most useful experimental and computational measures of plasticity for neurorehabilitation. Collaborations between researchers developing novel assays of plasticity and those using large clinical datasets to predict clinical outcomes, such as discussed by Liew, will be invaluable for future research and translation. Though such collaborations will likely involve navigating the challenges of measurement feasibility, such challenges highlight the potential promise of extending neurorehabilitation ‘in-the-wild’ (see below) and research into quantifying plasticity metrics.
Designing interventions that induce plasticity is central to any rehabilitation effort. Data-driven predictive models, such as those developed by Liew, provide methods for predicting how someone may respond to an intervention dose. However, these models have largely been used to predict a single endpoint, which may miss dynamic interactions between plasticity and an intervention, as highlighted by other presentations (e.g., Orsborn, Hight). Research with brain-computer interfaces and cochlear implants demonstrate that even interventions that intend to replace a function (rather than rehabilitate) induce plasticity. This plasticity may be influenced by how the device is designed (e.g., Orsborn’s investigations into co-adaptation with brain computer interfaces), and could be further manipulated by purposeful device interventions (e.g., vagus nerve stimulation presented by Hight). User-device interactions to shape plasticity open a huge opportunity to shape plasticity for rehabilitation. Capitalizing on this opportunity, however, will require improving models of how devices induce plasticity. Translating methods to shape plasticity with devices into meaningful clinical therapies will also require methods to predict functional outcomes.
Beyond these examples of scientific challenges, we also noticed important challenges and opportunities to create the scientific community needed to tackle these challenges. All talks focused on plasticity, but we were particularly struck by the topic diversity. For instance, the presentations spanned upper limb movements (Roth, Orsborn), locomotion (Mariscal), clinical sensorimotor function assessments (Liew), and hearing/speech (Hight). There was also a diverse range of methods used to quantify plasticity, from behavior (Roth, Mariscal, Liew), clinical neuroimaging (Liew), and high-resolution electrophysiology (Orsborn, Schwock). This breadth fostered rich discussions across sub-fields that do not regularly interact. Integrating the knowledge gained from this diversity of methods and applications and refining models of plasticity and adaptation for rehabilitation will require bridges across these communities and translating terminology between fields.
More at link.
No comments:
Post a Comment