You'll want your myelination tested post stroke. But further research required, testing in stroke patients to identify the amounts of demyelination and where they are.
What are your doctor's and hospital's EXACT PROTOCOLS for rebuilding your myelination post stroke? Don't have any? Then you don't have a functioning stroke doctor or hospital.
Human biodistribution and radiation dosimetry of the demyelination tracer [18F]3F4AP
European Journal of Nuclear Medicine and Molecular Imaging (2022)
Abstract
Purpose
[18F]3F4AP is a novel PET radiotracer that targets voltage-gated potassium (K+) channels and has shown promise for imaging demyelinated lesions in animal models of neurological diseases. This study aimed to evaluate the biodistribution, safety, and radiation dosimetry of [18F]3F4AP in healthy human volunteers.
Methods
Four healthy volunteers (2 females) underwent a 4-h dynamic PET scan from the cranial vertex to mid-thigh using multiple bed positions after administration of 368 ± 17.9 MBq (9.94 ± 0.48 mCi) of [18F]3F4AP. Volumes of interest for relevant organs were manually drawn guided by the CT, and PET images and time-activity curves (TACs) were extracted. Radiation dosimetry was estimated from the integrated TACs using OLINDA software. Safety assessments included measuring vital signs immediately before and after the scan, monitoring for adverse events, and obtaining a comprehensive metabolic panel and electrocardiogram within 30 days before and after the scan.
Results
[18F]3F4AP distributed throughout the body with the highest levels of activity in the kidneys, urinary bladder, stomach, liver, spleen, and brain and with low accumulation in muscle and fat. The tracer cleared quickly from circulation and from most organs. The clearance of the tracer was noticeably faster than previously reported in nonhuman primates (NHPs). The average effective dose (ED) across all subjects was 12.1 ± 2.2 μSv/MBq, which is lower than the estimated ED from the NHP studies (21.6 ± 0.6 μSv/MBq) as well as the ED of other fluorine-18 radiotracers such as [18F]FDG (~ 20 μSv/MBq). No differences in ED between males and females were observed. No substantial changes in safety assessments or adverse events were recorded.
Conclusion
The biodistribution and radiation dosimetry of [18F]3F4AP in humans are reported for the first time. The average total ED across four subjects was lower than most 18F-labeled PET tracers. The tracer and study procedures were well tolerated, and no adverse events occurred.
Background
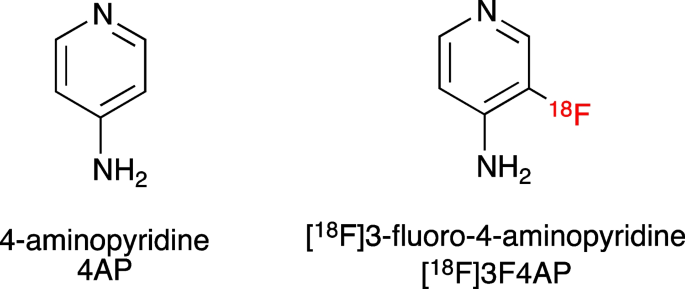
[18F]3-fluoro-4-aminopyridine ([18F]3F4AP) is a radiofluorinated analog of the multiple sclerosis (MS) drug 4-aminopyridine (dalfampridine, 4AP) [1] (Fig. 1). Similar to 4AP, [18F]3F4AP binds to voltage-gated K+ channels (Kv1 family) in demyelinated axons and has been proposed as a PET tracer for imaging demyelinated lesions in the brain. In demyelinated lesions, axonal K+ channels Kv1.1 and Kv1.2, which are normally buried under the myelin sheath and confined to the juxtaparanodal regions of the axons, become exposed and increase in expression [2,3,4]. This aberrant expression of K+ channels results in excessive efflux of intracellular K+ ions and impaired axonal conduction, thus causing neurological deficits in MS and other demyelinating diseases. The FDA-approved drug 4AP binds to and blocks the K+ channels in demyelinated axons, reducing the abnormal efflux of K+ ions from axons and partially restoring conduction [5,6,7,8,9]. Given the increase in axonal K+ channel expression and the ability of 4AP to bind to these channels, [18F]3F4AP, a radiofluorinated analog of 4AP, was proposed as a PET tracer for demyelination [1, 10]. [18F]3F4AP is similar to 4AP in that it can enter the brain by passive diffusion and bind to K+ channels in demyelinated axons. Previous studies in rodents showed that [18F]3F4AP can be used to detect lesions in a rat model of demyelination using PET [1]. Additional studies in rhesus macaques showed that [18F]3F4AP has suitable properties for imaging primate brains including high brain penetration, fast kinetics, minimal plasma protein binding, and high metabolic stability [11]. Furthermore, PET imaging of a monkey with a small focal traumatic brain injury sustained 3 years prior to imaging showed excellent sensitivity to the lesion [11]. These findings have prompted us to translate [18F]3F4AP to human research studies (Clinicaltrials.gov identifiers: NCT04699747, NCT04710550). As the first step in the evaluation of [18F]3F4AP in human subjects, we set out to assess the whole-body biodistribution, safety, and radiation dosimetry in healthy human volunteers. We also compare these results with previous findings in nonhuman primates.
No comments:
Post a Comment