Did your doctor and hospital do anything with this earlier research to get you recovered? Of course they didn't, they don't follow research at all.
intranasal administration (1 post to June 2022)
intranasal delivery (1 post to January 2023
Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting
1
Department of Mechanical and Aerospace Engineering, Politecnico di Torino, Corso Duca Degli Abruzzi 24, 10129 Turin, Italy
2
Interuniversity Center for the Promotion of 3Rs Principles in Teaching and Research, Centro 3R, 56122 Pisa, Italy
3
Institute for Chemical-Physical Processes, National Research Council (CNR-IPCF), 56124 Pisa, Italy
*
Author to whom correspondence should be addressed.
Int. J. Mol. Sci. 2023, 24(4), 3390; https://doi.org/10.3390/ijms24043390
Received: 30 December 2022
/
Revised: 22 January 2023
/
Accepted: 2 February 2023
/
Published: 8 February 2023
(This article belongs to the Special Issue Micro-Scale Approaches in Regenerative Medicine and Tissue Engineering 2022)
Abstract
Intranasal (IN) drug delivery is a non-invasive and
effective route for the administration of drugs to the brain at
pharmacologically relevant concentrations, bypassing the blood–brain
barrier (BBB) and minimizing adverse side effects. IN drug delivery can
be particularly promising for the treatment of neurodegenerative
diseases. The drug delivery mechanism involves the initial drug
penetration through the nasal epithelial barrier, followed by drug
diffusion in the perivascular or perineural spaces along the olfactory
or trigeminal nerves, and final extracellular diffusion throughout the
brain. A part of the drug may be lost by drainage through the lymphatic
system, while a part may even enter the systemic circulation and reach
the brain by crossing the BBB. Alternatively, drugs can be directly
transported to the brain by axons of the olfactory nerve. To improve the
effectiveness of drug delivery to the brain by the IN route, various
types of nanocarriers and hydrogels and their combinations have been
proposed. This review paper analyzes the main biomaterials-based
strategies to enhance IN drug delivery to the brain, outlining unsolved
challenges and proposing ways to address them.
1. Introduction
Pathologies
affecting the brain tissue, such as neurodegenerative diseases, brain
tumors and stroke, need the local administration of drugs for treatment.
However, the effectiveness of drug administration to the brain is
hampered by the presence of the blood–brain barrier (BBB), which is the
biological barrier that regulates the transport of molecules between the
blood and the brain. The BBB is constituted by the brain microvascular
endothelium (formed by a monolayer of endothelial cells) with its
basement membrane, pericytes surrounding the endothelium and astrocytes
mediating the interaction between neuronal cells and oligodendrocytes
with the brain capillaries. The BBB has a selective permeability, which
is fundamental for normal brain physiology [1,2]. The interplay among endothelial cells, pericytes and astrocytes regulates BBB integrity in vivo as well as in vitro [3,4].
Hence, the BBB protects the brain from the entry of potentially toxic
substances, however, it also prevents the delivery of therapeutics into
the central nervous system (CNS) for disease treatment. The BBB shows a
low level of pinocytosis and possesses tight junctions, which form a
seal between opposing endothelial membranes. The presence of tight
junctions causes a high transendothelial electrical resistance of
1500–2000 Ω·cm2 compared to 3–30 Ω·cm2 in the peripheral microvasculature [5].
For these reasons, the BBB highly restricts paracellular diffusion of
solutes from the blood into the brain. Typically, only small lipophilic
molecules may cross the BBB via transcellular passive diffusion,
although some limited transport of certain peptides and peptide analogs
has been reported [6].
Transcellular active diffusion occurs through specific transporters or
receptors, such as glucose transporter 1 for glucose and transferrin
receptor for iron [2,5].
In addition, receptors and transporters for gastrointestinal hormones
involved in regulating metabolism are expressed at the BBB in order to
convey information between CNS and peripheral parts of the body [5].
Besides the low paracellular diffusion and low rate of pinocytosis, the
endothelial layer of the BBB is also provided with efflux pumps (such
as P-glycoprotein) which further restrict the entry of substances that
would be otherwise predicted to cross the BBB based on their chemical
characteristics and molecular weight [7].
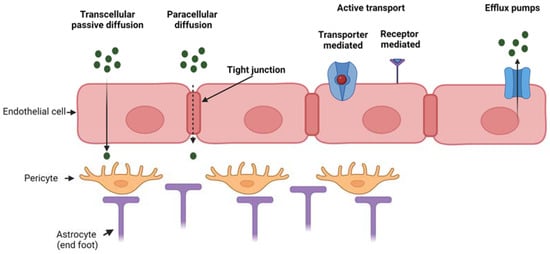
Figure 1 reports the main transport mechanisms across the endothelial layer of the BBB.
Figure 1.
Mechanism of transport through the BBB which is composed of three main
cell types (endothelial cells, pericytes and astrocytes). Passive
transport through transcellular or paracellular diffusion, active
transport (either transporter- or receptor-mediated) and efflux active
pumps. Created with BioRender.com.
Although a few low molecular weight drugs can
cross the BBB, high molecular weight hydrophilic substances are severely
restricted from crossing the BBB under normal conditions.
Intraparenchymal, intracerebroventricular and intrathecal
injections/infusions can be used to directly deliver therapeutics into
the CNS [8],
but these routes of administration are invasive and likely not
practical for drugs which need to be given frequently for the treatment
of chronic diseases.
Additional invasive
methods include the temporary disruption of BBB integrity, e.g., by
osmotic shock to the endothelial layer by mannitol administration or by
an ultrasound disruption technique [8].
Such methods are costly, require long-term hospitalization and are
associated with several drawbacks related to enhanced BBB permeability,
such as neuron damage by cytotoxic molecules crossing the BBB.
Alternative
strategies include a chemical modification of drugs to enhance their
lipophilicity without affecting their activity or the introduction of
hydrophilic molecules into nanomicelles with a hydrophobic shell, e.g.,
based on polaxamer-type copolymers [8].
Further “physiological approaches” are possible exploiting the
mechanisms involved in the transport of metabolites and catabolites
across the BBB, i.e., transcytosis mechanisms [8].
For example, it is possible to incorporate drugs within nanocarriers
surface functionalized with ligands interacting with insulin or
transferrin receptors on the endothelial cell layer of the BBB. One main
disadvantage is that such transcytosis mechanisms are not specific of
the BBB, therefore part of the drug-loaded nanocarriers may reach
different tissues than the CNS with possible side effects. Moreover,
systemic delivery of drug-loaded nanocarriers to reach the brain by
intravenous or oral administration is challenging due to the hepatic
first pass metabolism effect reducing drug half-life [9].
Hence,
intranasal (IN) delivery has emerged as a non-invasive and direct route
for drug administration to the brain bypassing the BBB and allowing
rapid brain localization [10].
The benefits afforded by IN delivery, especially the removal of
systemic side effects and the possibility to deliver biologics to the
brain (peptides, proteins, oligonucleotides and even cells), places it
as a potentially powerful route for brain disorder treatment [10].
However, the main limitations of IN delivery of drugs include: (i) loss
of non-absorbed drugs in the respiratory and digestive tracts with
potential side effects; (ii) rapid muco-ciliary clearance increasing
drug loss; (iii) low nasal epithelium permeability for macromolecular
and hydrophilic drugs; (iv) nasal mucosa metabolism of drugs by
proteolytic enzymes. To bypass these limitations, new advanced IN drug
delivery systems are required with the following characteristics: (i)
high encapsulation efficiency, (ii) ability to protect the drugs from
degradation/denaturation, (iii) ability to favor drug retention at the
nasal mucosa and (iv) ability to promote drug transport through the
nasal epithelium to reach the brain tissue. Several types of
nanoparticles and hydrogels have been developed to achieve these aims in
order to increase the effectiveness of the drug transport by the IN
route. This review is intended to explain the general mechanism for drug
delivery to the brain by the IN route, and how this has been enhanced
by the design and use of nanoparticles and hydrogels as drug carriers.
Research efforts aimed at improving efficiency of IN drug carrier
systems are discussed.
2. Overview of Nasal Anatomy
Since ancient time, drugs have been delivered though the nasal cavity for local and systemic drug delivery. For instance, in Ayurveda, the traditional Indian medicine, Nasya therapy, one of the Panchakarmas, is the process by which a medicine (in form of decoctions, oils and fumes) is intranasally administered [11].
The
nose allows the entrance of air into the body during respiration: its
main function is to filter, warm and humidify air. Moreover, the nose
provides an immunological barrier to protect the nasal cavity from
irritations and infections and it is the seat of the olfactory sense.
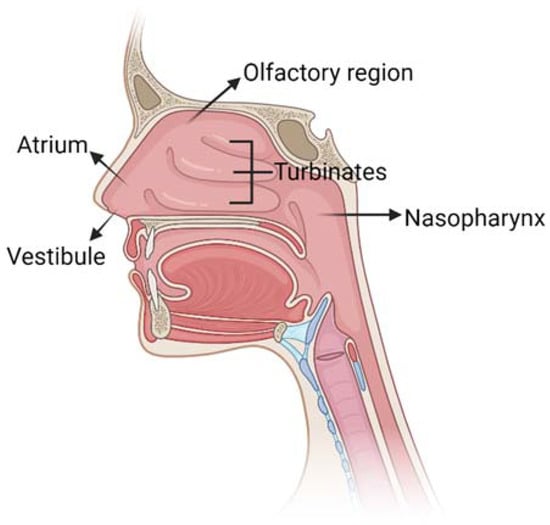
The nasal cavity has a total volume of 16–19 mL [12] and it is composed of five main regions (Figure 2): the vestibule, the atrium, the olfactory region, the respiratory zone and the nasopharynx.
Figure 2.
Schematic representation of nose anatomy with its five main regions:
vestibule, atrium, turbinates in the respiratory region, olfactory
region and nasopharynx. Created with BioRender.com.
The respiratory and olfactory regions are the
ones involved in IN drug delivery due to their superior permeability and
vascularization compared to the other nasal sites. The respiratory and
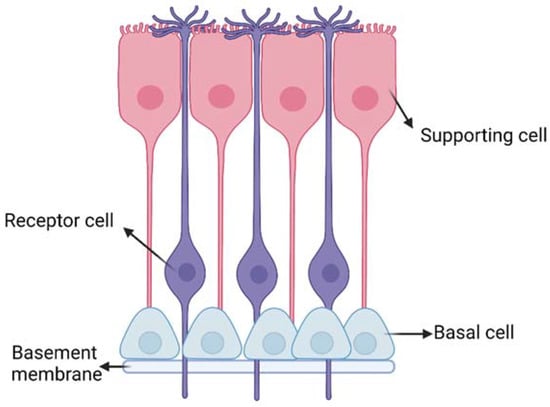
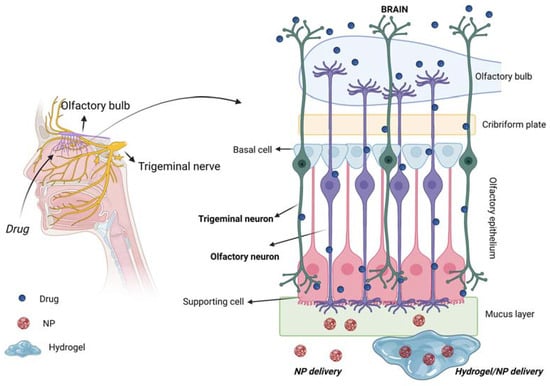
olfactory regions have an extent of around 160 cm2 and 15 cm2, respectively [5]. The olfactory epithelium is composed of different cells (Figure 3): (i) the olfactory receptor cells provided with non-motile cilia; (ii) the supporting cells and (iii) the basal cells [13].
Moreover, it is coated with a mucus layer produced by the Bowman’s
glands. The olfactory receptor cell is a bipolar neuron, forming an
amyelinated axon at its basal surface, conveying olfactory information
to brain. In its apical surface, each olfactory receptor develops a
unique dendritic process that expands into a protuberance with several
microvilli called olfactory cilia. Olfactory cilia are coated with a
mucus layer: when, during a cold, the mucus layer thickens, olfactory
sensibility decreases [14]. Amyelinic fibers and blood vessels run along the basal part of the mucosa, called lamina propria.
Figure 3.
Schematic representation of the olfactory epithelium with the three main
cells: (i) the olfactory receptor cells provided with non-motile cilia;
(ii) the supporting cells and (iii) the basal cells. Created with
BioRender.com.
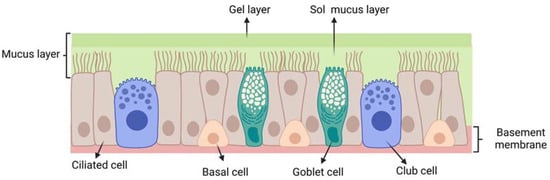
The respiratory epithelium (Figure 4)
is composed of: (i) ciliated pseudostratified columnar epithelial cells
(with around 100 cilia per cell); (ii) non-ciliated cells; (iii) goblet
cells and (iv) basal cells. Each ciliated and non-ciliated cell
possesses around 300 microvilli [15].
The respiratory epithelium is coated with a mucus layer and its cilia
are responsible for muco-ciliary clearance, the protective mechanism of
the respiratory system.
3. Mechanism for Drug Delivery to the Brain through the Intranasal Route
3.1. Mucus Layer: A First Barrier to Intranasal Drug Delivery
A
first barrier for intranasal drug transport is represented by the mucus
layer on the nasal epithelium. Mucus, mainly composed by mucin
polysaccharide, is a hydrogel layer with intrinsic porosity and negative
charges. Such features may slow down the drug diffusion rate through
the mucus layer. When the drug diffusion rate across the mucus layer is
lower compared to the muco-ciliary clearance rate, drug bioavailability
decreases. It has been estimated that the muco-ciliary clearance
half-time is around 15–30 min [16,17]. Clearance may be counteracted by the use of muco-adhesive drug carriers, as described in Section 5.
Furthermore,
the nasal cavity has a slightly acidic pH (5.5–6-5) and contains
enzymes that may catalyze the degradation of drugs, such as peptides and
proteins, thus reducing drug bioavailability [18].
Typical approaches to address such issues are the use of enzyme
inhibitors or drug doses above the saturation concentration of enzymes [19].
3.2. Transport of Drugs from Nasal Epithelium to the Brain
The
precise pathways and mechanisms by which a drug travels from the nasal
epithelium to various regions of the brain have not been fully
elucidated. Lochhead et al. [5] and Thorne et al. [20] have described such mechanisms. Studies on [125I]-labeled
proteins following IN administration in rats and monkeys have shown
that delivery occurs along the olfactory and trigeminal nerve components
in the nasal epithelium to the olfactory bulb and brainstem,
respectively, with further dispersion to other brain areas [20]. At least three sequential transport steps are necessary for drugs to be delivered to the brain following IN administration [5].
3.2.1. Transport across the Olfactory and Respiratory Epithelial Barriers
Transport across the olfactory or respiratory epithelia may occur either by intracellular or extracellular mechanisms.
Intracellular
pathways are activated by the olfactory sensory neurons, the trigeminal
nerve and the nasal epithelial cells and include two different
mechanisms depending on the involved cells: (i) endocytosis by the
neural cells on the nasal epithelial surface and subsequent
intraneuronal transport; (ii) transcytosis (i.e., transcellular
transport) across the cells of the respiratory and olfactory epithelium
to the lamina propria.
Extracellular transport
pathways include paracellular diffusion across the olfactory and the
respiratory epithelia to the underlying lamina propria. Paracellular
transport depends on the presence of tight junctions in the olfactory
and respiratory epithelia: tight junction tightness and continuity
determine the permeability to paracellular transport. It has been
suggested that the regular turnover of cells in the nasal epithelium may
lead to continuous rearrangement and loosening of tight junctions which
favors paracellular transport of drugs [5].
3.2.2. Transport from the Nasal Mucosa to the Sites at Brain Entry
This
transport may occur via intracellular pathways (intraneuronal transport
through endocytosis within olfactory sensory neurons or trigeminal
ganglion cells) or extracellular pathways (diffusion or convection
within perineural, perivascular or lymphatic channels, associated with
olfactory or trigeminal nerve bundles extending from the lamina propria
to the brain). In the case of substances reaching the extracellular
space, different fates are possible: (i) absorption into blood vessels,
entering the systemic circulation; (ii) absorption into lymphatic
vessels reaching neck cervical lymph nodes; (iii) extracellular
diffusion or convection in perineural or perivascular nerve bundles
spaces, leading to access to the cranial site. Drugs absorbed into the
systemic circulation should cross the BBB or blood–cerebrospinal fluid
(CSF) barrier to reach the brain. Hence, the nasal vasculature may act
as a sink hindering some molecules from reaching the brain. Drugs that
have reached the lamina propria and escaped the local absorption into
the blood stream and drainage within nasal lymphatics may enter the
brain tissue. Studies have shown that drugs can be transported in the
spaces of the perineural sheath surrounding the olfactory nerve [5].
Moreover, although tight junctions are present, olfactory ensheathing
cells maintain open spaces for the regrowth of olfactory nerve fibers,
creating an additional extracellular path that substances may take to
reach the brain, along with entering the olfactory nerve bundles.
Finally, the perineural spaces of cranial nerves, such as the olfactory
and trigeminal nerves, appear to allow communication with CSF of the
subarachnoid space for some substances, providing an additional route
for molecules to reach the brain [5].
3.2.3. Transport from the Initial Brain Entry Sites to Other Brain Areas
Once
the drug has reached the olfactory bulb and brainstem, it is
distributed to other brain areas by two distinct possible mechanisms:
(i) intracellular transport, by drug transfer to neurons forming a
synapsis with peripheral olfactory sensory neurons or trigeminal
ganglion cells; (ii) extracellular transport, through distribution
within the cerebral perivascular spaces into the parenchyma. For
instance, in the case of [125I]-labeled proteins such as
insulin-like growth factor 1 (IGF-1) and interferon-β1b (INF-β1b), a
convective mechanism within perivascular spaces of the cerebral blood
vessels caused their distribution to different brain sites [20].
Expansion and contraction of perivascular spaces with the cardiac cycle
may generate a pronounced fluid flow within them under normal
conditions. Although modeling studies have been performed to understand
the direction and characteristics of this flow, different results have
been obtained and predictions of drug distribution in the brain are
still a challenge [21,22].
However, increased blood pressure and heart rate have been demonstrated
to improve the intraparenchymal distribution of large substances via
the perivascular spaces [23].
Another study has also found that the rostral migratory stream, the
pathway used by neuronal progenitors to migrate from perivascular
regions to the olfactory bulb, may also play a role in the delivery of
molecules from the nasal cavity into the brain [24].
Among
the possible transport mechanisms to the brain, the direct axonal
transport has been found to be incompatible with the short measured time
taken by intranasally administered drugs to reach the brain [25]. Such findings have demonstrated that direct axonal transport is not the main route for IN drug delivery to the brain.
3.3. Synthesis of the Main Features of Intranasal Drug Transport to the Brain
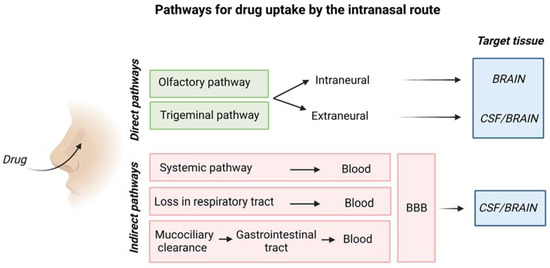
Figure 5 is a schematic representation of the overall mechanism for IN drug delivery to the brain.
Figure 5.
Schematic representation of drug uptake by the intranasal route. Created with BioRender.com.
The olfactory pathway conveys drugs directly
to the olfactory bulb, whereas the trigeminal nerve enters the CNS in
the pons transporting drugs to cerebrum and cerebellum [26].
Table 1 describes advantages and disadvantages of the IN drug delivery route.
Table 1.
Summary of main advantages and disadvantages of the IN route for drug delivery to the brain.
The degree of drug targeting to the brain
after intranasal (IN) administration with respect to intravenous (IV)
administration can be assessed by the drug targeting efficiency (DTE %)
and the direct transport percentage (DTP %) [26]:
AUC is the area under the drug concentration–time curve.
BIN is the AUC0–24h (brain) following intranasal administration.
BX is the brain AUC fraction from systemic circulation after intranasal administration.
BIV is the AUC0–24h (brain) following intravenous administration.
PIN is the AUC0–24h (blood) following intranasal administration.
PIV is the AUC0–24h (blood) following intravenous administration.
DTE%
is used to indicate the tendency of the drug to accumulate in the brain
when administered via the IN route compared to IV administration.
Values above 100% evidence a higher efficacy of the IN compared to IV
route. DTP% refers to the quantity of drug entering the brain through
direct pathways (i.e., trigeminal and olfactory pathways). When DTP% is
higher than zero, drug brain targeting is reached via the direct
pathways, while for values below 0 the IV route is more effective
compared to IN administration. Finally, a DTP% of 100 can only be
achieved if the drug is not adsorbed by the blood circulation after IN
administration (i.e., PIV: AUC0–24h (blood) is zero) or if the drug is not able to cross the BBB (i.e., BIV: AUC0–24h (brain) is zero) [27,28].
Alternatively,
the percentage of drug accumulation in the brain (bioavailability, B%)
can be evaluated considering only the AUC data for the brain areas,
without including AUC for blood:
As for the DTE, a higher accumulation of the drug in the brain is represented by B% values above 100.
When
nanocarriers are employed, the relative bioavailability (RB%) can be
evaluated, comparing the IN drug delivery through nanoparticles with
respect to the delivery of the drug in solution form.
Values of RB% above 100 indicate a
higher accumulation of drug in the brain with the use of the
nanocarriers compared to the “naked” drug.
Moreover,
RDTE% and RDTP% can also be utilized to evaluate the effect of the
nanocarriers in drug administration through the IN route.
It has been found that an efficient
delivery to the brain by the intranasal route also depends on the head
position, type of used formulation and delivery device [10].
3.4. Prevalent Transport Mechanisms for Hydrophilic Drugs by the Intranasal Route
Intranasally
administered drugs mainly cross the nasal epithelium barrier by a
transcellular or paracellular mechanism (rather than by direct axonal
transport) and, then, a portion of them is successfully transported to
the brain, by diffusion in the perivascular or perineural spaces along
the olfactory or trigeminal nerves. However, transport mechanisms depend
on drug chemistry. A paracellular mechanism is possible for polar drugs
with molecular weight lower than 1000 Da, allowing the diffusion
thorough the 10 Å sized channels of transiently opened tight junctions
in the nasal epithelium [29].
A polar drug with molecular weight higher than 1000 Da, such as
polypeptides and proteins, can pass the nasal membrane by endocytic
transport, although in low amounts [29].
For high molecular weight hydrophilic drugs, absorption enhancers can
be used, including surfactants, bile salts and their derivatives, fatty
acids and their derivatives, phospholipids, various cyclodextrins and
cationic molecules (e.g., poly(lysine) or chitosan and its derivatives) [29],
as explained in detail in the next section. Briefly, absorption
enhancers increase the permeability of the epithelial cell membrane by
different mechanisms depending on the type of enhancer, for example,
increasing transcellular transport [29].
Moreover, they can also improve paracellular transport by promoting the
transient opening of tight junctions. However, due to their interaction
with the nasal epithelium layer, absorption enhancers can elicit
cytotoxic effects towards the nasal mucosa, especially in the case of
repeated drug administration for the treatment of chronic diseases [30,31].
4. Penetration Enhancers in IN Drug Delivery
Penetration
enhancers have been proposed for IN drug delivery formulations: they
can be components of the drug carriers or additives of the drug
formulations, and can be used alone or in combination to exploit
synergistic effects. A range of surfactants have been investigated using
both synthetic (e.g., sodium lauryl-sulfate) and naturally derived
(e.g., BC9-BS biosurfactant isolated from Lactobacillus gasseri BC9)
materials to improve drug penetration by inducing membrane rupture or
improving the fluidity of the membrane, then favoring transcytosis [32,33]. Non-ionic alkyl saccharide surfactants, including dodecyl maltoside (Intravail®),
have also been associated with the possibility to enhance drug
penetration by transcellular transport involving cellular
internalization of drugs into vesicles, although the mechanism of action
is still under investigation [34]. Nonetheless, dodecyl maltoside is commercially available in US FDA-approved nasal sprays for migraine (Tosymra®) and seizure clusters (Valtoco®).
Cationic
polymers (e.g., chitosan or poly-L-arginine) have been shown to
interact with the negatively charged cellular residues, reducing
transepithelial resistance and promoting tight junction opening, which
affects paracellular transport [35,36].
Tight junctions opening can also be favored by a reduction of
endogenous calcium ions through the use of calcium chelators (e.g.,
ethylenediaminetetraacetic acid, EDTA) or anionic poly(acrylic acid)
polymers (e.g., Carbapol®) able to bind cations [33,37].
Finally, the use of peptide-mimicking toxins has also been investigated
to modulate the function of tight junctions. The C-terminal fragment of
an enterotoxin derived from the Gram-positive bacterium Clostridium perfringens
(C-CPE) has been shown to increase paracellular transport of nasal
pneumococcal vaccine by binding with claudins, proteins involved in
tight junction formation [38,39]. The peptide AT1002 is an analog of Vibrio cholera toxin, acting on zonula occludens in tight junctions. Peptide AT1002 enhances IN permeation by binding its receptor reversibly and opening tight junctions [40].
Mucolytic
agents constitute another class of penetration enhancers for IN
delivery able to decrease the viscosity of mucus. N-acetyl-l-cysteine is
a widely used mucolytic agent able to cleave the disulfide bonds
responsible for mucin fiber crosslinking, increasing mucus mesh size and
permeability [18].
5. Biomaterials-Based Vehicles for IN Drug Delivery
Biomaterials-based
delivery systems, consisting of microparticles, nanoparticles and
hydrogels, offer the possibility to improve the efficiency of intranasal
drug delivery to the brain, as they may protect drugs from degradation,
increase their absorption into and transport across the nasal
epithelium (while decreasing drug loss in the respiratory and digestive
tracts during administration) and—in the case of tailor-designed
nanoparticles—favor brain targeting.
As mucus
represents the first physical barrier for drug absorption by the nasal
mucosa, specific chemical and physical features of the mucus layer have
to be taken into account for the design of efficient IN drug delivery
systems. A thin mucus layer of approximately 10–15 µm in thickness
covers the nasal epithelium, consisting of a highly hydrated polymeric
network secreted by the goblet cells in the mucosa, mainly composed by
water (around 95 wt.%) and mucins (2–5 wt.%), glycoproteins containing
sialic acid units. Previous studies have reported different values of
mucus hydrogel mesh size (50–1800 nm), however, the average size of
mucus intrinsic pores is considered to be 20–200 nm [41].
Besides water and mucins, mucus also contains various amounts of DNA,
plasma proteins, immunoglobulins (particularly secretory IgA), lysozyme,
lactoferrin, lipids and polysaccharides [41]. The pH of mucus is slightly acidic (pH 5.5–6.5) [42].
Furthermore, mucus is continuously propelled towards the pharynx by the
cilia present in the respiratory mucosa, at a rate of 5 mm/min [43] with an average clearance time of around 15–30 min [16,17].
Muco-adhesivity, i.e., the ability to attach to the mucus, has been
frequently proposed as a key feature for efficient polymeric drug
carriers in the IN route. Different theories have been exploited to
explain muco-adhesivity, as reviewed by Khutoryanskiy et al. [42].
In brief, muco-adhesion can arise from electrostatic interactions
between positively charged materials in contact with negatively charged
mucins (electronic theory). Additionally, hydrogen and van der Waals
bonding or hydrophobic interactions can be established contributing to
muco-adhesion (absorption theory). Based on the wetting theory,
biomaterial adhesion to mucus layer is promoted by the ability of the
drug formulation to wet and spread over the mucus layer. According to
the diffusion theory, an interpenetration between macromolecules and the
mucin network is responsible for muco-adhesion. Furthermore, the
mechanical theory predicts that muco-adhesion depends on the contact
area between the biomaterial and the mucus layer, which in turns depends
on the carrier surface roughness. Finally, the fracture theory predicts
that muco-adhesion is due to chemical compatibility between mucus and
the biomaterial, leading to an interface resistant to relative
detachment. Based on that, muco-adhesivity is typically a property of
polymers with positively charged chains, such as chitosan, able to
interact with negatively charged mucins or able to form hydrogen bonds
or other secondary interactions (e.g., hydrophobic interactions) with
mucins. On the other hand, non-adhesive materials include antifouling
polymers, such as poly(ethylene glycol) (PEG). A review article by
Sosnik et al. has discussed the main muco-adhesive polymers for drug
delivery, including chitosan, cellulose derivates and poly(acrylic acid)
and poly(methacrylic acid) derivatives [41].
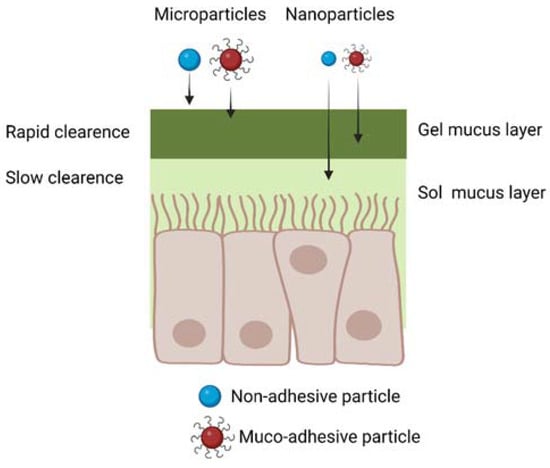
5.1. Microparticles and Nanoparticles for IN Drug Delivery
Drug-loaded
polymer microparticles, having sizes larger than 500 nm, are a
suboptimal choice for IN drug delivery, as they are unable to diffuse
through the smaller nanometric pores of the mucus layer (Figure 6).
However, muco-adhesive microparticle with sizes in the 500 nm–10 µm
range, escaping the filtration by the nasal vestibule, have been
proposed for IN drug delivery, exploiting their ability to bind to the
mucus layer [44,45].
Their drug delivery efficiency depends on the ability of loaded drugs
to diffuse through the microparticles and the nasal mucus layer,
reaching the nasal epithelium in a shorter time than that required for
muco-ciliary clearance. Furthermore, in this application, drugs should
be resistant to degradation and able to cross the nasal epithelium,
without the help of biomaterial carriers.
Figure 6.
Schematic representation of micro- and nanoparticle diffusion through
the nasal mucus considering both muco-adhesive and non-muco-adhesive
particles. Created with BioRender.com.
On the other hand, drug-loaded nanoparticles
are more advantageous than microparticles, due to their ability to
penetrate and diffuse through the nasal mucus (Figure 6), and to support all the subsequent phases of drug transport to the brain, previously described in Section 3.
Muco-adhesive nanoparticles have been frequently proposed for IN drug
delivery, as they attach to the nasal mucus after administration,
decreasing the possibility for their loss in the respiratory and
digestive tracts [45,46].
On the other hand, muco-adhesive nanoparticles show limited drug
delivery efficiency: as they slowly diffuse across the mucus layer, they
are partially lost during the muco-ciliary clearance mechanism. As a
solution, antiadhesive nanoparticles weakly interact with the mucus
layer: although this property increases the quantity of nanoparticles
lost in the digestive and respiratory systems post-administration, it
increases nanoparticle diffusion rate across mucus. For this reason,
antiadhesive nanoparticles have also been proposed for IN drug delivery [47,48,49,50].
Table 2 collects exemplary types of nanocarriers exploited for IN drug delivery.
Table 2
evidences that nanocarriers for IN drug delivery have been prepared
from both natural materials and synthetic polymers. Among natural
polymers, gelatin nanoparticles have been widely employed due to their
biocompatibility, biodegradability, low immunogenicity and possibility
for surface functionalization [70].
Frequently, gelatin nanoparticles have been prepared in combination
with Poloxamer 188 to reduce mucus viscosity and elasticity, and
modulate tight junction opening [58,71]. Chitosan nanoparticles have also been widely exploited for IN drug delivery [36,72].
Chitosan is a cationic polysaccharide able to induce tight junction
opening, favoring paracellular transport of drugs when used in the form
of nanoparticles, hydrogel or nasal solution [36,72].
Among
synthetic polymers, poly(lactic-co-glycolic acid) (PLGA) copolymer has
been widely investigated as a biocompatible and biodegradable material
for the production of numerous drug delivery formulations, enabling
encapsulation and controlled release of both hydrophobic and hydrophilic
drugs [73]. Chitosan coating of PLGA-based nanoparticles has been mainly investigated to introduce muco-adhesive properties [55,56].
Lipid-based
nanocarriers have also been investigated for IN drug delivery thanks to
their low toxicity, biocompatibility, biodegradability and flexibility.
In particular, second-generation lipid carriers, solid lipid
nanocarriers (SLNs) and nanostructured lipid carriers (NLPs) have been
mainly studied [56,62,63,69].
SLNs are composed of a solid lipid core containing the drug stabilized
by surfactants, while NLPs possess a solid lipid core with a surfactant
outer shell. SLNs and NLPs have shown higher drug loading, better drug
stability and prolonged release profile compared to liposomes [74,75].
Finally, exosomes, naturally derived nanocarriers secreted by cells,
have recently attracted interest as drug delivery systems for the
treatment of brain disorders through IN administration thanks to their
low immunogenicity, good biocompatibility, possibility of
functionalization for targeted action and broad spectrum of endogenous
and exogenous bioactive cargo molecules, including protein, nucleic
acids, growth factors and therapeutic agents [65,67,76]. Interestingly, the nanocarrier surface has been frequently functionalized with:
- Muco-adhesive polymers such as chitosan or polymer containing thiol groups (thiomers);
- Molecules for adsorption endocytosis by the epithelial layer (e.g., lectins, cell-penetrating peptides, such as penetratin, Tat peptide, etc.);
- Molecules for ligand-mediated endocytosis (e.g., lactoferrin);
- Mucus-penetrating (non-adhesive) polymers (e.g., PEG).
Muco-adhesive
functionalities have been used to decrease the muco-ciliary clearance
time, increasing IN drug release efficiency. In some cases, the
muco-adhesive molecules have additional functionalities, e.g., chitosan
is able to enhance drug passage through the nasal mucosa by transiently
opening the tight junctions connecting the epithelial cells as
previously underlined. Thiomers have also been investigated for their
muco-adhesive properties thanks to their interactions with cysteine
residues of mucus glycoproteins [60,77].
Lectins are proteins able to reversibly bind mono-sugars or oligosaccharides, promoting both muco-adhesion and endocytosis [78,79].
Different types of lectins have been tested for surface
functionalization of nanoparticles for IN drug delivery. Gao et al.
reported the conjugation of WGA onto PEG-PLA particles [52,80],
showing their enhanced brain targeting ability with respect to
unfunctionalized particles, or intranasally or intravenously
administered drug solutions. Gao et al. have also conjugated wheat germ
agglutinin to PEG-PLA particles as it binds to L-fucose in the olfactory
epithelium, enhancing drug release to the brain [52]. Chen et al. coupled STL to PLGA nanoparticles with a 1.89–2.45-fold increase in brain targeting [81].
STL-conjugated PEG-PLGA nanoparticles loaded with basic fibroblast
growth factor (bFGF) enhanced brain delivery compared to intravenous
injection of bFGF, and IN administration of bFGF solution and
bFGF-loaded PEG-PLGA nanoparticles. The superior DTP % values of
STL-conjugated PEG-PLGA nanoparticles indicated that more than 70% of
the drug was directly delivered to the brain by the IN route [26].
Cell penetrating peptides have shown enhanced nose-to-brain transport [82,83]. Wan et al. have identified specific peptides for nose-to-brain drug delivery by the phage display method [82]. Yang et al. prepared liposomes loaded with rivastigmine and surface functionalized with a cell-penetrating peptide [64].
Zhai et al. modified the surface of exosomes with rabies virus
glycoprotein (RVG) peptide able to specifically bind to neuronal
acetylcholine receptor for the delivery of brain-derived neurotrophic
factor for multiple sclerosis applications [67].
Finally, Peng et al. proposed a novel nanocarrier system, characterized
by a polymer micellar core composed of PPS−PEG, encapsulated in an
exosome outer shell modified with penetratin and RVG peptides for the
delivery of curcumin for Parkinson’s disease treatment [66].
Lactoferrin
is a natural protein binding iron, that has the ability to interact
with the lactoferrin receptors that are abundant on respiratory
epithelial cells. The functionalization of drug-loaded nanoparticles
with lactoferrin allows their absorption by epithelial cells through a
transcytosis mechanism. Liu et al. prepared PEG-co-poly(ε-caprolactone)
nanoparticles surface functionalized with lactoferrin and incorporating
NAP (NAPVSIPQ) a neuroprotective peptide [51].
As
mentioned above, non-muco-adhesive properties have also been
investigated to develop systems able to penetrate through the mucus
barrier without adhering to it [49,50].
PEGylation is the main modification investigated to develop
mucus-penetrating systems. However, the molecular weight and density of
charge are two main parameters that affect the adhesion ability of PEG,
as recently reviewed by Lai et al. [84].
Low molecular weight PEG combined with a high PEG density favors
penetration, due to the generation of an almost neutral surface able to
minimize mucus interactions. On the contrary, high molecular weight PEG
and a low density of coatings have been shown to induce muco-adhesive
properties [46,84].
Porfiryeva et al. showed the higher penetration of PEGylated
derivatives of polyelectrolyte complexes formed by oppositely charged
Eudragits (i.e., anionic Eudragit® L100-55 and cationic Eudragit® EPO) compared to non-modified complexes [54].
Ways et al. investigated the possibility to create mucus-inert chitosan
nanoparticles through grafting with PEG, PHEA, POZ and PVP. All the
modified nanocarriers showed superior mucus penetration compared to
unmodified chitosan with PVP showing the highest penetration depth in ex
vivo sheep nasal mucosa [48].
5.2. Hydrogels for IN Drug Delivery
Hydrogels
are three-dimensional crosslinked networks of hydrophilic polymers able
to absorb and retain a considerable amount of water without dissolving,
preserving their shape [85].
Hydrogels for IN drug delivery have been generally designed to be
muco-adhesive in order to bind to the nasal mucosa. Hydrogels may also
undergo some mixing with the mucus layer, facilitating drug penetration [86].
Moreover, IN administration of hydrogels affects the viscosity of the
mucus–hydrogel systems, increasing the muco-ciliary clearance time and
enhancing the effectiveness of IN drug uptake [87].
Finally, hydrogels can shield drugs from undergoing chemical and
enzymatic degradation in the nasal cavity, prolonging their activity.
Hydrogels
are generally sprayed into the nasal cavity or administered as solution
drops, converting into a hydrogel upon contact with the nasal mucosa.
Stimuli-responsive hydrogels have been generally used for IN drug
delivery exploiting the following physical features of the nasal cavity:
(1) pH 5.5–6.5; (2) temperature of 32 °C; (3) presence of sodium,
calcium and potassium ions [86].
Hence, thermosensitive and pH- and ion-responsive hydrogels have been
proposed for IN drug delivery as reviewed by Chonkar et al. and
Protopapa et al. [86,88].
Table 3 collects exemplary types of promising hydrogel formulations for drug delivery to the brain by the IN route developed to date.
5.3. Nanocarrier-Loaded Hydrogels for IN Delivery
As discussed in Section 5.1,
both antiadhesive and muco-adhesive nanoparticles have been proposed
for IN drug delivery. However, the potential advantages of muco-adhesive
nanoparticles have not been confirmed by in vivo biodistribution
trials. Indeed, studies in mouse models have shown that muco-adhesive
chitosan-coated nanostructured lipid nanocarriers, loaded with protein
drugs, although effective for drug delivery to the brain, mainly
accumulated in the lungs, followed by the liver, the kidneys, the spleen
and, finally, the brain [68].
These findings have prompted criticisms on the real benefits of
muco-adhesive nanoparticles for IN drug delivery to the brain, as such
nanoparticles could more easily reach different tissues with potential
side effects.
To overcome these drawbacks, more
complex pharmaceutical formulations could be exploited, based on
hydrogels releasing drug-loaded nanoparticles. Both weakly muco-adhesive
hydrogels, such as Poloxamer [98,99], and highly muco-adhesive hydrogels, such as chitosan, Poloxamer/gellan and Poloxamer/chitosan [100,101,102,103,104], have been proposed. Such formulations are collected and described in Table 4.
The hydrogel should improve local nanoparticle retention, decreasing
losses in the digestive and respiratory tracts. Furthermore, hydrogels
may increase the muco-ciliary clearance time and improve nanoparticle
uptake by the nasal epithelium.
Table 4.
Exemplary nanoparticle-loaded hydrogel formulations investigated for IN drug delivery to reach the brain.
Although different types of nanoparticles have
been administered in combination with hydrogels, nanoparticles based on
lipids and/or natural polymers are affected by limited stability and
can be more easily disassembled/degraded in vivo with respect to
nanoparticles based on synthetic polymers [105,106].
Hence, the type of hydrogel embedding nanoparticles should be carefully
selected to avoid unfavorable interactions with lipid- or natural
polymer-based nanoparticles, leading to their disaggregation in contact
with the hydrogel.
Figure 7
schematically shows the routes for drug transport to the brain of
intranasally administered drugs, mediated by the use of the
above-described biomaterials (i.e., various types of NPs, hydrogels and
NP/hydrogel systems). The olfactory and trigeminal pathways are
highlighted as being the two main direct routes for drug delivery to the
brain.
6. Discussion
IN
delivery represents a direct route for effective drug administration to
the brain, particularly suitable for hydrophilic high molecular weight
drugs, such as growth factors, which are not able to cross the BBB and
can induce side effects in the peripheral tissues when systemically
administered.
Kozlovskaya et al. analysed 73
publications between 1970 and 2014 concerning the quantitative analysis
of the delivery of drugs or model agents to the brain via IN and
parenteral routes. According to this literature investigation, the use
of nanoparticles or hydrogel carriers for IN drug delivery has offered
limited advantages with respect to drug solutions. On the other hand,
compounds able to favor drug delivery through the IN route, such as
absorption enhancers muco-adhesive compounds and targeting ligands have
shown a higher impact on drug delivery effectiveness of IN therapy [107].
Particularly,
drug-loaded nanoparticles have a key role in protecting drugs from
enzymatic degradation and in efficiently transporting them across the
nasal barrier to the brain through proper surface functionalities. Drugs
crossing the nasal barrier are partially absorbed by the systemic
circulation and hence not able to reach the brain (Figure 5).
Their encapsulation into intranasally administered nanoparticles,
provided with the additional ability to cross the BBB, could increase
the efficiency of drug release to the brain. Additionally, nanoparticles
could be functionalized with specific ligands potentially favoring drug
release to specific brain cells. Among nanoparticles prepared from
natural polymers or their derivatives (Table 2), chitosan-based nanoparticles have been widely used for IN delivery due to their muco-adhesive properties [36,72].
However, they do not have the intrinsic ability to cross the BBB or to
reach target brain cells, unless properly functionalized [108].
Due to their hydrophilicity and consequent limited stability in
physiological media, chitosan nanoparticles cannot be easily surface
functionalized after their preparation. On the other hand, surface
functionalization could be achieved through complex and laborious
methods involving chemical derivatization of chitosan molecules before
nanoparticle preparation [109].
One additional drawback of chitosan nanoparticles for protein or
polypeptide drug encapsulation is the use of acidic solution for their
preparation, with the risk of drug denaturation/degradation [110].
Similarly, nanocarriers provided with a lipid shell and a gelatin core
are among the most widely exploited for IN drug delivery: they can be
prepared in mild conditions and the lipid shell favors cell
internalization (Table 3) [57,58]. However, their stability could be limited by rapid in vivo disassembly and gelatin degradation by proteolytic enzymes [75,111,112].
Unfunctionalized lipid–gelatin nanoparticles have been generally used
for IN drug delivery, however, their surface functionalization with
targeting ligands could be achieved by the use of previously synthesized
ligand-functionalized lipids [113]. PEG-polyester nanocarriers (Table 2)
represent another class of nanoparticles widely used for IN drug
delivery, due to their biocompatibility, biodegradability and
antifouling properties [51,52].
However, they also show a few limitations when applied for the release
of protein drugs: (i) proteins can be loaded into PEG-polyester
nanoparticles by emulsion methods, using organic solvents, which may
affect protein bioactivity; (ii) surface functionalization of
PEG-polyester nanoparticles with targeting ligands generally requires
their incubation into functionalizing solutions, potentially causing
partial protein drug release and/or denaturation/degradation; (iii)
degradation of PEG-polyester nanoparticles by bulk hydrolysis may cause
denaturation/degradation of loaded proteins before they reach their
therapeutic target [114].
Overall,
state-of-the-art analysis carried out in this review paper has shown
that optimal nanocarriers for IN delivery of drugs (particularly
proteins and polypeptides) to the brain have not yet been designed.
Requirements for optimal nanoparticles for IN drug delivery to the brain
include: high encapsulation efficiency, preparation using mild
conditions, suitable size for diffusion through the intrinsic pores of
the mucus layer, stability in contact with mucus and blood, antifouling
surface functionalities (to facilitate diffusion through the mucus)
coupled with specific ligands favoring transport through the nasal
epithelium and subsequent brain targeting. One additional requirement is
nanoparticle stability in the presence of an additional hydrogel
exploited to increase IN drug delivery efficiency. Indeed, the
combination of drug-loaded nanocarriers and hydrogels could enhance drug
absorption by the nasal mucosa, decreasing the amount of nanocarriers
lost in the gastrointestinal and respiratory systems. However, previous
research studies have suggested that hydrogels need to be properly
designed to avoid the risk of slowing down the diffusion ability of
polymer nanocarriers to reach the nasal epithelium, with a consequent
decrease in nanocarrier uptake [115].
Intermolecular interactions between the nanocarrier surface and the
hydrogel should be weak while hydrogel (as well as hydrogel/mucus) mesh
size should be larger compared to nanocarrier diameter.
Based
on our recent research work (unpublished), we have found that polymer
nanocarriers with a neutral surface are generally stable and undergo
sustained release from hydrogels at a rate depending on
nanocarrier–hydrogel reciprocal interactions and mesh size. Conversely,
positively charged nanocarriers should be embedded in hydrogels with
neutral charge to avoid their collapse within the hydrogel with
consequent premature cargo release. Hydrogel composition should also be
selected considering its possible positive effect as an enhancer for
nanocarrier uptake by the nasal epithelium.
More broadly, the design of efficient systems for IN drug delivery to the brain should ensure:
- Therapeutic concentrations of drugs to the brain within a short time from the administration (~30 min), with prompt therapeutic benefits for the patients.
- Improved drug bioavailability avoiding hepatic first pass metabolism.
- Reduced side effects due to the lack of accumulation into non-target tissues, such as the liver, or the possibility to avoid gastroprotective drugs (needed for orally administered drugs in the treatment of chronic diseases).
- Patient-compliant treatment exploiting a minimally invasive route.
- Reduction of therapy costs due to enhanced effectiveness including brain-targeting ability.
- Avoidance of local and systemic toxicity, which is frequently associated with long-term treatment of chronic diseases.
- Improvement of patient’s quality of life, by an effective drug administration route.
IN
therapy could be applied for the treatment of several brain
pathologies, including neurodegenerative diseases, stroke and brain
tumors. To date, IN drug formulations for specific treatment of brain
pathologies are missing on the market or still at the stage of clinical
trials. One major example is represented by the IN drug delivery of
insulin, investigated in the SNIFF clinical trials as a possible
treatment for cognitive defects in patients affected by Alzheimer’s
disease (clinicaltrials.gov: NCT03857321, NCT00438568, NCT01767909).
Clinical trials have shown side effects of IN delivery of insulin
formulations, such as nosebleeds and rhinitis [116].
Indeed, all the tested insulin formulations (Detemir, Humalog, Apidra,
etc.) made use of excipients, such as cresol, meta-cresol and phenol,
responsible for rhinitis, nosebleeds and allergic reactions [117].
Additionally, insulin formulations have shown effectiveness in
improving the cognition of adults with mild cognitive impairment or
early-stage Alzheimer’s disease [118].
On the contrary, they have failed to impact on cognition in individuals
with mild–moderate Alzheimer’s disease carrying the ε4 allele of
apolipoprotein E, an established risk factor for late-onset Alzheimer’s
disease [119,120].
These findings suggest that although insulin may have some therapeutic
effect in the Alzheimer’s disease treatment, improved IN insulin
delivery formulations are needed. Phase 2 and 3 controlled trials have
been recently completed, investigating the efficacy of two IN insulin
devices (ViaNase by Kurve Technology and I109 Precision Olfactory
Delivery by Impel NeuroPharma) on patients with mild cognitive
impairment or Alzheimer’s disease over 18 months. Preliminary results
suggested a better performance of the ViaNase device, showing higher
cognitive performance scores and Alzheimer’s disease biomarker
production. However, possible differences in the dosage delivered by the
two systems might have led to the discrepancies in the results [121,122],
leading to the set-up of a new clinical trial, currently ongoing, to
evaluate the specific dosage of insulin delivered by the devices (SNIFF
Device, clinicaltrials.gov: NCT01767909).
One
main problem in the design of intranasal drug delivery formulations is
their preclinical validation through poorly predictive in vivo mouse and
rat models [123,124].
Recently, physiologically relevant in vitro models of the human nasal
epithelium have been developed and are commercially available for
preclinical investigations. For example, EpiNasal™ by MatTek Life
Sciences mimics the nasal epithelium structure by an air–liquid
interface coculture of epithelial cells and mucus-producing goblet cells
with functional tight junctions and beating cilia. Moreover, Gholizadeh
et al. developed a first nasal epithelial mucosa (NEM)-on-a-chip model
able to measure in real time the barrier integrity (obtained through
transepithelial electrical resistance, TEER, measurements) and the drug
transport (e.g., ibuprofen transport, chosen as a model agent) across a
human nasal cell layer cultured at the ALI interface in both static and
dynamic (via pulsatile systemic circulation in the basolateral
compartment) conditions [125].
Capuana et al. developed a perfusion bioreactor system mimicking the
nasal mucosa, characterized by a scaffold with an inner channel allowing
cells to be in contact with both cell culture medium and air under
dynamic conditions [126].
However, micro- or milli-fluidic devices recapitulating drug transport
through the nose to reach the brain would be required to improve the
design of optimal drug formulations for IN drug delivery to the brain,
allowing better preclinical validation studies and reduction in the use
of animal experimentation, according to the principles of replacement,
reduction and refinement (the 3Rs).
7. Conclusions
This
review paper highlighted the advantages of IN drug delivery systems,
for their potential ability to provide a direct, rapid and effective
route for drug delivery to the brain for the treatment of cancer,
neurodegenerative diseases, stroke and other pathologies (e.g.,
migraine). The knowledge of the routes taken by intranasally
administered drugs, together with the main biological barriers involved
in the transport, is fundamental for the design of biomaterials-based
carriers able to efficiently deliver drugs to the brain. Although
different delivery vehicles have been proposed, more research is needed
to improve drug delivery efficiency, decreasing side effects, both
locally and systemically. Multifunctional formulations based on
hydrogels encapsulating drug-loaded nanocarriers could represent the
solution and their composition should be optimized based on the
physicochemical properties of drugs, nanocarrier release kinetics and
optimal adsorption by the nasal epithelium. These efforts require the
parallel development of improved in vitro models for accurate
preclinical evaluation before clinical trials.
The
increasing incidence of brain pathologies as a consequence of
progressive aging makes urgent the need for effective IN drug delivery
nanoformulations. IN drug delivery systems targeting the brain tissue
have the potentiality to address societal challenges, providing
effective treatments for age-related diseases, including
neurodegenerative diseases and brain cancers.



No comments:
Post a Comment