Does your doctor or anyone on the stroke medical world have the ability to see the possibilities of using this for stroke? Since stem cells create exosomes and exosomes seem to be the helping factor for recovery.
Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations May2021
The latest here
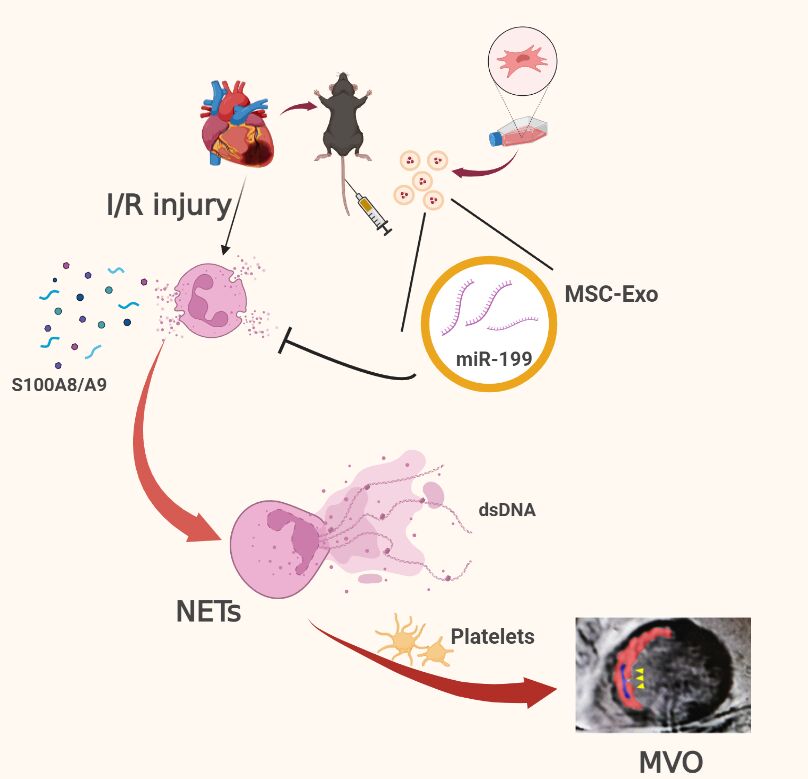
MSC-Derived Exosomes Mitigate Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil Infiltration and the Formation of Neutrophil Extracellular Traps
Authors Feng Y, Bao X, Zhao J, Kang L, Sun X, Xu B
Received 19 September 2023
Accepted for publication 14 February 2024
Published 5 March 2024 Volume 2024:19 Pages 2071—2090
DOI https://doi.org/10.2147/IJN.S436925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Yuting Feng, Xue Bao, Jinxuan Zhao, Lina Kang, Xuan Sun, Biao Xu
Department
of Cardiology, Nanjing Drum Tower Hospital, State Key Laboratory of
Pharmaceutical Biotechnology, Medical School of Nanjing University,
Nanjing, People’s Republic of China
Correspondence: Xuan Sun;
Biao Xu, Department of Cardiology, Nanjing Drum Tower Hospital, State
Key Laboratory of Pharmaceutical Biotechnology, Medical School of
Nanjing University, No. 321 Zhongshan Road, Nanjing, 210008, People’s
Republic of China, Email sunxuan891119@163.com; xubiao62@nju.edu.cn
Introduction:
Acute inflammatory storm is a major cause of myocardial
ischemia/reperfusion (I/R) injury, with no effective treatment currently
available. The excessive aggregation of neutrophils is correlated with
an unfavorable prognosis in acute myocardial infarction (AMI) patients.
Exosomes derived from mesenchymal stromal cells (MSC-Exo) have certain
immunomodulatory potential and might be a therapeutic application.
Therefore, we investigated the protective role of MSC-Exo in modulating
neutrophil infiltration and formation of neutrophil extracellular traps
(NETs) following myocardial I/R injury.
Methods:
Exosomes were isolated from the supernatant of MSCs using a gradient
centrifugation method. We used flow cytometry, histochemistry, and
immunofluorescence to detect the changes of neutrophils post-intravenous
MSC-Exo injection. Additionally, cardiac magnetic resonance (CMR) and
thioflavin S experiments were applied to detect microvascular
obstruction (MVO). The NLR family pyrin domain containing 3 (NLRP3)
inflammasome was examined for mechanism exploration. Primary neutrophils
were extracted for in vitro experiment. Antibody of Ly6G was given to
depleting the neutrophils in mice for verification the effect of
MSC-Exo. Finally, we analyzed the MiRNA sequence of MSC-Exo and verified
it in vitro.
Results: MSC-Exo administration
reduced neutrophil infiltration and NETs formation after myocardial I/R.
MSC-Exo treatment also could attenuate the activation of NLRP3
inflammasome both in vivo and in vitro. At the same time, the infarction
size and MVO following I/R injury were reduced by MSC-Exo. Moreover,
systemic depletion of neutrophils partly negated the therapeutic effects
of MSC-Exo. Up-regulation of miR-199 in neutrophils has been shown to
decrease the expression of NETs formation after stimulation.
Discussion:
Our results demonstrated that MSC-Exo mitigated myocardial I/R injury
in mice by modulating neutrophil infiltration and NETs formation. This
study provides novel insights into the potential therapeutic application
of MSC-Exo for myocardial ischemia/reperfusion injury.
Keywords: myocardial ischemia/reperfusion injury, mesenchymal stromal cells, exosomes, neutrophil extracellular traps, mir-199
Graphical Abstract:
Introduction
Acute myocardial infarction (AMI) has been a major cause of death and heart failure (HF) worldwide in current. Percutaneous coronary intervention (PCI) to recanalize the artery in the early stage of AMI salvages the injured myocardium and improves survival. However, the rapid restoration of blood flow triggers ischemia/reperfusion (I/R) injury,1 which reduces the curative effect of PCI.
The inflammatory response is one of the important pathological mechanisms of myocardial I/R injury.2 Neutrophils play a pivotal role in mediating inflammation as the first immune cells recruited from the bloodstream into injured tissues.3 The number and proportion of neutrophils in the peripheral blood and myocardium tissue increase rapidly in the acute phase of I/R injury and the infiltration of neutrophils in the myocardium reaches the peak within 24 hours after reperfusion. Neutrophils remove necrotic debris at the injured site and recruit the mononuclear macrophage system for further repair.4 But the excessive aggregation and activation of neutrophils result in the release of a plethora of damage-associated molecular patterns (DAMPs) and inflammatory cytokines, which subsequently contribute to myocardial necrosis, aggravated ventricular remodeling, and reduced cardiac function.5
Activated neutrophils can release neutrophil extracellular traps (NETs), manifesting as weblike DNA structures decorated with histones and antimicrobial proteins.6 The released NETs bind to platelets and red blood cells and then finally form plugs. These plugs are dispersed by blood flow into microvessels, forming embolisms that impede tissue perfusion despite the restoration of large coronary arteries.7 This phenomenon, known as microvascular obstruction (MVO), is a major contributor to I/R injury and infarct size, and an increased risk of developing heart failure after AMI.8 Besides, NETs promote the formation of reactive oxygen species9 and the secretion of inflammatory factors and chemokines to aggravate the inflammatory response.10 Neutrophils have also been proven as the primary source of the proinflammatory alarmins, S100A8 and S100A9, after AMI.11 The release of S100A8 and S100A9 in the myocardium stimulates additional leukocyte recruitment and increases the secretion of pro-inflammatory cytokines.12 High plasma levels of S100A8 and S100A9 after PCI are associated with poor left ventricular ejection fraction (LVEF) and an increased incidence of HF13 in AMI patients. These pathological processes above finally result in a vicious circle of myocardium injury. Neutrophil influx and NETs are associated with poor outcomes after cardiac ischemia. However, there are currently not any clinically approved interventions able to modulate neutrophils after I/R injury.
Mesenchymal stromal cells (MSCs) have been demonstrated to have immunomodulatory properties14 and the therapeutic applications of MSCs are being explored in cardiovascular diseases.15 However, the risk of thrombosis formation and immunoreactivity have suggested that caution is still warranted in the clinical use of MSCs.16 The exosomes secreted by MSCs (MSC-Exo) have been known to mediate cell-to-cell communication, which has well-established anti-inflammatory effects.17 We have reported that MSC-Exo plays a protective effect on ischemic myocardium by polarizing inflammatory macrophage towards an anti-inflammatory macrophage population.18 In consideration that neutrophils infiltration into the myocardium peaks within 24 hours after reperfusion and they are prime drivers of the pro-inflammatory phase, it remains unknown whether MSC-Exo could inhibit neutrophils from mobilization, recruitment or MVO formation thus limiting inflammation.
In this study, we assessed whether MSC-Exo therapy in the early stage could suppress neutrophil infiltration and NETs formation to preserve cardiac function. Our research has shown the promising effect of MSC-Exo in myocardium I/R injury.
No comments:
Post a Comment