http://www.medscape.org/viewarticle/760409
Introduction
People with coronary stents are among the many cardiovascular patients prescribed long-term antiplatelet therapy. Dual antiplatelet therapy, comprising a P2Y12 inhibitor (usually clopidogrel) and aspirin, is recommended for a minimum of 12 months after drug-eluting stent placement.[1] Premature cessation of dual antiplatelet therapy in patients after acute coronary syndrome (ACS) and/or those treated with drug-eluting stents is associated with an increased risk for cardiovascular events, including stent thrombosis which can be fatal.[2]
Although this antiplatelet combination reduces the risk for ischemic events compared with aspirin alone, it is also associated with significantly more major bleeding.[3] In the VALsartan In Acute myocardial iNfarcTion trial (VALIANT) study of almost 15,000 survivors of myocardial infarction, serious gastrointestinal bleeding occurred in 0.7% over a median 2 years. Use of dual antiplatelet therapy was the most powerful predictor of this adverse event and serious gastrointestinal (GI) bleeding was associated with an increased risk of death [adjusted hazard ratio 2.54 (95% CI; 1.66-3.89)].[4] Smaller bleeds occur more frequently and long-term antiplatelet therapy is also associated with dyspepsia and other forms of GI distress.[5] Thus, proton pump inhibitors (PPIs) are often used in patients on long-term antiplatelet therapy.
Pharmacodynamic and Pharmacokinetic Interactions
Clopidogrel is a prodrug that is metabolized to its active form in a 2-step process through the cytochrome P450 (CYP) pathway with the majority of it being hydrolyzed to an inactive derivative. The CYP2C19 isoenzyme is involved in both steps (Figure 1). Most PPIs available in the United States are also metabolized via the CYP system.
View abbreviations used in this activity.
Please read the introduction, then watch Dr Cryer's video commentary that includes a patient case simulation.
Introduction
People with coronary stents are among the many cardiovascular patients prescribed long-term antiplatelet therapy. Dual antiplatelet therapy, comprising a P2Y12 inhibitor (usually clopidogrel) and aspirin, is recommended for a minimum of 12 months after drug-eluting stent placement.[1] Premature cessation of dual antiplatelet therapy in patients after acute coronary syndrome (ACS) and/or those treated with drug-eluting stents is associated with an increased risk for cardiovascular events, including stent thrombosis which can be fatal.[2]
Although this antiplatelet combination reduces the risk for ischemic events compared with aspirin alone, it is also associated with significantly more major bleeding.[3] In the VALsartan In Acute myocardial iNfarcTion trial (VALIANT) study of almost 15,000 survivors of myocardial infarction, serious gastrointestinal bleeding occurred in 0.7% over a median 2 years. Use of dual antiplatelet therapy was the most powerful predictor of this adverse event and serious gastrointestinal (GI) bleeding was associated with an increased risk of death [adjusted hazard ratio 2.54 (95% CI; 1.66-3.89)].[4] Smaller bleeds occur more frequently and long-term antiplatelet therapy is also associated with dyspepsia and other forms of GI distress.[5] Thus, proton pump inhibitors (PPIs) are often used in patients on long-term antiplatelet therapy.
Pharmacodynamic and Pharmacokinetic Interactions
Clopidogrel is a prodrug that is metabolized to its active form in a 2-step process through the cytochrome P450 (CYP) pathway with the majority of it being hydrolyzed to an inactive derivative. The CYP2C19 isoenzyme is involved in both steps (Figure 1). Most PPIs available in the United States are also metabolized via the CYP system.
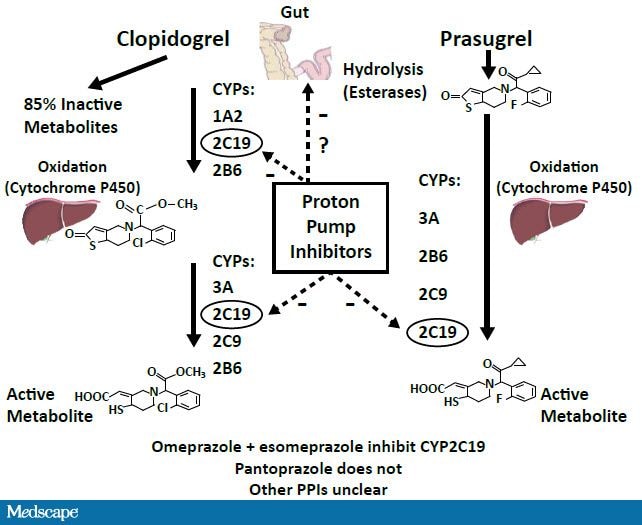 In vitro studies using assays to measure metabolite levels or platelet reactivity show that co-administration of PPIs and clopidogrel results in reduced active metabolite generation and weak inhibition of platelet reactivity.[6-7]
In vitro studies using assays to measure metabolite levels or platelet reactivity show that co-administration of PPIs and clopidogrel results in reduced active metabolite generation and weak inhibition of platelet reactivity.[6-7]
This interaction persists with spaced administration of delayed-release omeprazole and clopidogrel.[8] However, spaced administration (10 hours) of an investigational drug combining enteric-coated delayed-release aspirin (325 mg) with immediate release omeprazole (40 mg) mitigated any pharmacodynamic interaction with clopidogrel.[9] It is not clear whether these pharmacodynamic and pharmacokinetic interactions hamper the clinical efficacy of clopidogrel.
Clinical Events
Observational studies show a higher risk for cardiovascular events in clopidogrel-treated patients co-administered PPIs, notably omeprazole.[10] Large randomized studies comparing clopidogrel with newer P2Y12 inhibitors, prasugrel in the TRITON-TIMI 38 (Trials to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel -- Thrombolysis in Myocardial Infarction) trial or ticagrelor in the PLATO (PLATelet inhibition and patient Outcomes) trial have yielded conflicting findings. The TRITON-TIMI 38 study did not show higher event rates in patients assigned clopidogrel who were taking PPIs compared with those on clopidogrel and not taking PPIs, nor did it show any effect in a similar analysis of patients assigned prasugrel.[11] However, in the PLATO trial, patients on PPIs in both the clopidogrel and ticagrelor groups had worse outcomes compared with their counterparts who were not taking PPIs.[12] That any interaction was seen in the ticagrelor-treated group was surprising since ticagrelor is a direct acting P2Y12 inhibitor that does not require biotransformation. PPI use was not randomized in these trials and patients on these gastroprotective drugs tended to be higher risk at baseline, confounding the data.
To date, the Clopidogrel and Optimization of Gastrointestinal Events (COGENT) study is the only antiplatelet trial to randomize PPI use. Almost 3800 patients with an indication for dual antiplatelet therapy were randomized to a novel clopidogrel/omeprazole combination pill or clopidogrel alone; all patients were taking aspirin. The COGENT trial planned to enroll 5,000 patients but it was stopped early because the supporter and manufacturer of the clopidogrel/omeprazole pill filed for liquidation. The trial showed no difference in ischemic events between the 2 groups; indeed, those taking PPIs experienced significantly fewer upper GI bleeding events.[13]
Please read the introduction, then watch Dr Cryer's video commentary that includes a patient case simulation.
Introduction
People with coronary stents are among the many cardiovascular patients prescribed long-term antiplatelet therapy. Dual antiplatelet therapy, comprising a P2Y12 inhibitor (usually clopidogrel) and aspirin, is recommended for a minimum of 12 months after drug-eluting stent placement.[1] Premature cessation of dual antiplatelet therapy in patients after acute coronary syndrome (ACS) and/or those treated with drug-eluting stents is associated with an increased risk for cardiovascular events, including stent thrombosis which can be fatal.[2]
Although this antiplatelet combination reduces the risk for ischemic events compared with aspirin alone, it is also associated with significantly more major bleeding.[3] In the VALsartan In Acute myocardial iNfarcTion trial (VALIANT) study of almost 15,000 survivors of myocardial infarction, serious gastrointestinal bleeding occurred in 0.7% over a median 2 years. Use of dual antiplatelet therapy was the most powerful predictor of this adverse event and serious gastrointestinal (GI) bleeding was associated with an increased risk of death [adjusted hazard ratio 2.54 (95% CI; 1.66-3.89)].[4] Smaller bleeds occur more frequently and long-term antiplatelet therapy is also associated with dyspepsia and other forms of GI distress.[5] Thus, proton pump inhibitors (PPIs) are often used in patients on long-term antiplatelet therapy.
Pharmacodynamic and Pharmacokinetic Interactions
Clopidogrel is a prodrug that is metabolized to its active form in a 2-step process through the cytochrome P450 (CYP) pathway with the majority of it being hydrolyzed to an inactive derivative. The CYP2C19 isoenzyme is involved in both steps (Figure 1). Most PPIs available in the United States are also metabolized via the CYP system.

This interaction persists with spaced administration of delayed-release omeprazole and clopidogrel.[8] However, spaced administration (10 hours) of an investigational drug combining enteric-coated delayed-release aspirin (325 mg) with immediate release omeprazole (40 mg) mitigated any pharmacodynamic interaction with clopidogrel.[9] It is not clear whether these pharmacodynamic and pharmacokinetic interactions hamper the clinical efficacy of clopidogrel.
Clinical Events
Observational studies show a higher risk for cardiovascular events in clopidogrel-treated patients co-administered PPIs, notably omeprazole.[10] Large randomized studies comparing clopidogrel with newer P2Y12 inhibitors, prasugrel in the TRITON-TIMI 38 (Trials to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel -- Thrombolysis in Myocardial Infarction) trial or ticagrelor in the PLATO (PLATelet inhibition and patient Outcomes) trial have yielded conflicting findings. The TRITON-TIMI 38 study did not show higher event rates in patients assigned clopidogrel who were taking PPIs compared with those on clopidogrel and not taking PPIs, nor did it show any effect in a similar analysis of patients assigned prasugrel.[11] However, in the PLATO trial, patients on PPIs in both the clopidogrel and ticagrelor groups had worse outcomes compared with their counterparts who were not taking PPIs.[12] That any interaction was seen in the ticagrelor-treated group was surprising since ticagrelor is a direct acting P2Y12 inhibitor that does not require biotransformation. PPI use was not randomized in these trials and patients on these gastroprotective drugs tended to be higher risk at baseline, confounding the data.
To date, the Clopidogrel and Optimization of Gastrointestinal Events (COGENT) study is the only antiplatelet trial to randomize PPI use. Almost 3800 patients with an indication for dual antiplatelet therapy were randomized to a novel clopidogrel/omeprazole combination pill or clopidogrel alone; all patients were taking aspirin. The COGENT trial planned to enroll 5,000 patients but it was stopped early because the supporter and manufacturer of the clopidogrel/omeprazole pill filed for liquidation. The trial showed no difference in ischemic events between the 2 groups; indeed, those taking PPIs experienced significantly fewer upper GI bleeding events.[13]
No comments:
Post a Comment