I've only heard of tDCS(transcranial direct current stimulation) instead of tACS(transcranial alternating current stimulation) so this is new to me. Your doctor can explain which is better.
HD-tDCS (3 posts)
cathodal tDCS (6 posts)
anodal tDCS (9 posts)
tDCS (61 posts)
Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation
Nature Neuroscience volume 25, pages 1237–1246 (2022)
Abstract
The development of technologies to protect or enhance memory in older people is an enduring goal of translational medicine. Here we describe repetitive (4-day) transcranial alternating current stimulation (tACS) protocols for the selective, sustainable enhancement of auditory–verbal working memory and long-term memory in 65–88-year-old people. Modulation of synchronous low-frequency, but not high-frequency, activity in parietal cortex preferentially improved working memory on day 3 and day 4 and 1 month after intervention, whereas modulation of synchronous high-frequency, but not low-frequency, activity in prefrontal cortex preferentially improved long-term memory on days 2–4 and 1 month after intervention. The rate of memory improvements over 4 days predicted the size of memory benefits 1 month later. Individuals with lower baseline cognitive function experienced larger, more enduring memory improvements. Our findings demonstrate that the plasticity of the aging brain can be selectively and sustainably exploited using repetitive and highly focalized neuromodulation grounded in spatiospectral parameters of memory-specific cortical circuitry.
Main
The world is facing many challenges due to a rapidly aging global population. The shift in age demographics is associated with considerable personal, social, healthcare and economic costs1. A critical factor contributing to aging-induced costs is the impairment in basic memory systems essential for activities of daily living, such as making financial decisions or comprehending language2. Emerging reports suggest an increased likelihood of such impairments due to the ongoing Coronavirus Disease 2019 (COVID-19) pandemic3. Moreover, there exists considerable variability in memory decline across individuals during aging4, with accelerated decline potentially predicting subsequent Alzheimer’s disease and other dementias5. Substantial progress in neuroscience has identified the brain circuits and networks that underpin memory capacities, and studies have suggested that the rhythmic activity of cognitive circuitry may be important for the coordination of information processing6. What is needed now are technologies to non-invasively isolate and augment the rhythmic activity of neural circuits, inspired by models of healthy aging, to determine whether it is possible to protect or even enhance memory function for older adults in a rapid and sustainable fashion6,7.
A challenge in improving memory function in older adults is that memory function may not be instantiated by a single cognitive mechanism. Previous research has characterized a capacity-limited working memory (WM) store for brief maintenance of information and an unlimited long-term memory (LTM) store for sustained maintenance of information8. Within this dual-store framework, previous research has identified both concurrent deficits9 and selective deficits10 in WM and LTM function with aging, using the classic immediate free recall paradigm, associating these stores with the canonical recency and primacy effects, respectively11. Neuropsychological research has long alluded to distinct anatomical and functional substrates of primacy and recency effects and the corresponding WM and LTM stores11,12,13. Differential contributions of the dorsolateral prefrontal cortex (DLPFC) and the inferior parietal lobule (IPL) have been suggested14. However, it is not known whether distinct rhythmic mechanisms in these regions subserve distinct memory processes during free recall. If unique rhythmic mechanisms in spatially distinct brain regions can be identified, then these brain rhythms can be independently and non-invasively manipulated using techniques such as high-definition transcranial alternating current stimulation (HD-tACS) for selectively improving memory function in older adults.
Rhythmic activity in the theta and gamma frequency ranges are thought to contribute to both WM15 and LTM16 function, particularly during free recall17. However, previous attempts at modulating these rhythms to improve memory have yielded inconsistent findings. Although there are some suggestions of improvements in WM with modulation of parietal theta rhythms18, changing theta rhythms in the frontal regions7,19 and gamma rhythms in the parietal20 and frontal21 regions have yielded contradictory results. Similarly, although frontal gamma tACS has previously suggested improvements in LTM22,23, other spatiospectral combinations, such as frontal theta24,25 and parietal theta26 modulation, have shown variable effects. In addition, although modulation of gamma rhythms in the medial parietal cortex has shown some benefits to LTM27, causal evidence for involvement of these rhythms in lateral parietal cortices is scarce. Moreover, much of this evidence comes from studies in young adults, using paradigms targeting visuospatial memory and using conventional tACS, which has poorer spatial resolution and target engagement than techniques such as HD-tACS guided by current flow models28. Thus, which specific combinations of location and frequency of neuromodulation are effective for selectively improving WM and LTM function, particularly in older adults, are unknown.
Based on the balance of evidence, we tested the hypotheses that modulation of theta rhythms in the IPL would improve auditory–verbal WM function (recency effect), whereas modulation of gamma rhythms in the DLPFC would improve auditory–verbal LTM function (primacy effect) in older adults (Experiment 1). To modulate these rhythms, we applied tACS with optimal source-sink configurations of nine 12-mm ring electrodes (8 × 1 tACS) guided by current flow models to improve the focality of current flow28. Moreover, we sought to induce long-lasting effects by performing repetitive neuromodulation over multiple days and tested memory performance up to 1 month after intervention. Furthermore, we examined the effect of interindividual differences4 and tested whether older individuals with lower general cognitive performance would benefit more from neuromodulation. To confirm the location specificity and frequency specificity of our hypotheses and address the conflicting findings in the field, we performed a second experiment (Experiment 2) in which we switched the entrainment frequencies in the two regions to examine the effect of gamma entrainment in the IPL and theta entrainment in the DLPFC on memory function. To explicitly test the replicability of the principal findings, we performed a third experiment (Experiment 3), similar to Experiment 1, examining the effect of gamma modulation in the DLPFC and theta modulation in the IPL in an independent sample of participants. Across these three experiments, we sought evidence for a double dissociation in the two memory stores according to the distinct spatiospectral characteristics of their underlying anatomical and functional substrates and, consequently, for selective and long-lasting improvements in memory function in older adults.
Results
We conducted a randomized, double-blind study consisting of two sham-controlled experiments to target memory function in older adults and an additional experiment to test the replicability of the principal findings. In Experiment 1, 60 participants (Table 1) were randomized into three groups (sham, DLPFC gamma and IPL theta; Fig. 1). We used a repetitive neuromodulation protocol in which each participant received 8 × 1 tACS according to their assigned group for 20 minutes each day on four consecutive days. Gamma frequency 8 × 1 tACS was administered at 60 Hz, whereas theta frequency 8 × 1 tACS was administered at 4 Hz, following previous studies suggesting stronger benefits at these frequencies18,22. On each day, participants performed five runs of the free recall task. In each run, they encoded a list of 20 words and were asked to immediately recall the words at the end of the presentation of the list. Neuromodulation was performed through the entire duration of encoding and recall of all five lists to increase functional specificity29, and this procedure took approximately 20 minutes (Methods). We examined memory performance across the five runs as a function of the serial position of the presented words. This allowed us to isolate changes in LTM and WM, separately, indexed by the primacy and recency serial position curve effects according to dual-store models11. In addition to these online assessments, we evaluated memory performance offline, at baseline and at 1 month after intervention. We also determined general cognitive function, quantified using the Montreal Cognitive Assessment (MoCA)30, and depression symptoms, assessed using the Geriatric Depression Scale (GDS)31, at baseline. Experiment 2 served as a control to test the frequency specificity of the effects in Experiment 1. Here, we switched the neuromodulation frequency between the two regions of interest. Sixty older participants (Table 1) were randomized into three groups (sham, DLPFC theta and IPL gamma; Fig. 1) and proceeded similarly to Experiment 1. Experiment 3 served as a test for replication of the primary findings from Experiment 1. Here, a new sample of 30 participants was randomized into the two critical conditions of interest from Experiment 1 (DLPFC gamma and IPL theta) and received neuromodulation for only three consecutive days; as in Experiment 1, we examined memory performance at baseline and during each neuromodulation session.
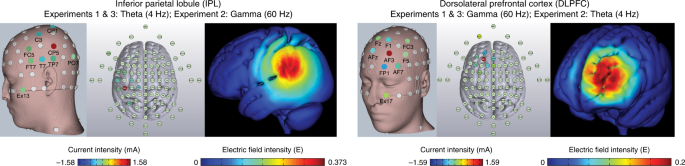
The theta-rate IPL and gamma-rate DLPFC HD-tACS protocols and corresponding electric field models shown on three-dimensional reconstructions of the cortical surface. The left DLPFC and left IPL were targeted, each protocol using nine electrodes configured in a center-surround, source-sink pattern to achieve maximum focality. The location and current intensity value of each modulating electrode are shown. The DLPFC protocol included (in mA): FP1 (−0.6662), Fz (0.0739), F1 (−0.4438), AF3 (1.5892), FC3 (−0.0048), F5 (−0.2312), AF7 (−0.194), AFz (−0.3744) and EX17 (0.2513). The IPL protocol included (in mA): C3 (−0.2997), T7 (−0.3386), CP1 (−0.2975), FC5 (−0.1284), CP5 (1.5818), FT7 (−0.0852), TP7 (−0.1413), PO7 (−0.2366) and EX13 (−0.0545).
DLPFC gamma modulation selectively improves LTM
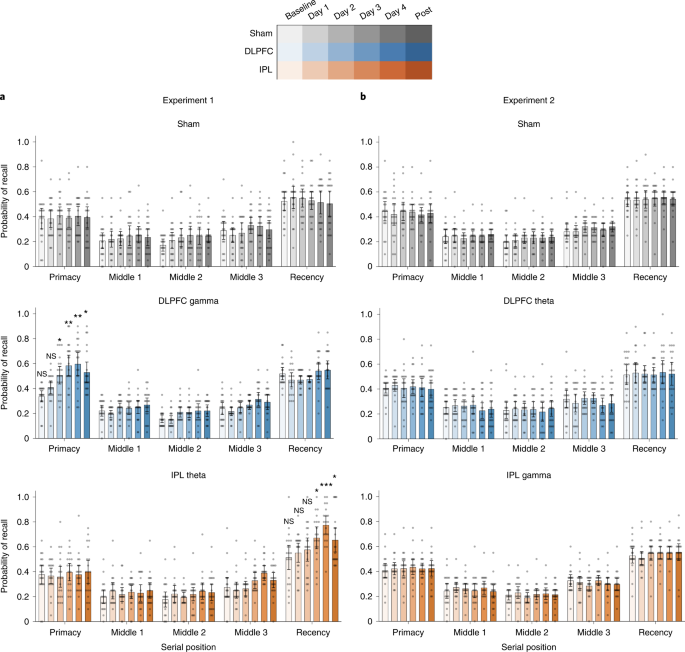
In Experiment 1, free recall performance across the five word lists administered during neuromodulation was averaged and entered into a mixed ANOVA with day (baseline, day 1, day 2, day 3, day 4 and 1 month after intervention) and serial position (primacy, middle 1, middle 2, middle 3 and recency) as within-subjects factors and group (sham, DLPFC gamma and IPL theta) as a between-subjects factor. We observed a significant day × serial position × group interaction (F21.4,611.5 = 3.875, P < 0.001, ηp2 = 0.120). A follow-up mixed ANOVA examining performance between the sham and DLPFC gamma groups showed a similar day × serial position × group interaction effect (F10.1,384.0 = 3.064, P < 0.001, ηp2 = 0.087). Additional follow-up analyses testing the effect of day on the serial position × group interaction showed that the differences in the sham and DLPFC gamma groups were present on day 2 (F3.3,126.8 = 7.228, P < 0.001, ηp2 = 0.160), day 3 (F2.9,110.3 = 15.331, P < 0.001, ηp2 = 0.287), day 4 (F2.8,107.0 = 10.698, P < 0.001, ηp2 = 0.220) and 1 month after intervention (F2.6,100.5 = 3.435, P = 0.024, ηp2 = 0.083). Examining the effect of serial position on the day × group interaction, we observed significant improvements in memory performance for the primacy cluster in the DLPFC gamma group with respect to sham (F3.6,140.4 = 7.470, P < 0.001, ηp2 = 0.164) and no differences in any other serial position cluster (Fs < 2.262, ps > 0.085). Parsing the improvements in the primacy cluster, independent-sample t-tests revealed significantly higher primacy performance in the DLPFC gamma group relative to the sham group on day 2, day 3, day 4 and 1 month after intervention (Fig. 2a, top, middle). The pattern of results remained unchanged when accounting for additional factors such as age, sex, years of education, MoCA and GDS scores as covariates (Supplementary Tables 1–3). Exploratory analyses suggested potentially greater improvements in males than females, but these effects did not survive correction for multiple comparisons (Extended Data Fig. 1). The results suggest that rhythmic neuromodulation in the gamma band targeting left DLPFC preferentially improved LTM in older adults. The improvements were rapidly induced by the second day of neuromodulation, persisted on all following neuromodulation days and lasted for at least 1 month after intervention.
A mixed ANOVA was performed to examine differences in recall probabilities in each experiment with the following factors: day (baseline, days 1–4 and 1 month), serial position (primacy, middles 1–3 and recency) and groups (E1: sham, DLPFC gamma and IPL theta; E2: sham, DLPFC theta and IPL gamma). Interaction effects were parsed with follow-up ANOVAs and two-sided independent-sample t-tests. a, Mean recall probabilities plotted across serial position clusters (primacy, three middles and recency) at pre-intervention baseline, day 1, day 2, day 3, day 4 and 1 month after intervention for Experiment 1 groups: sham (top, grays, n = 20), DLPFC gamma (middle, blues, n = 20) and IPL theta (bottom, oranges, n = 20) neuromodulation groups. Gray dots show individual participant data. Mean of center shows the average recall probability, and the error bars show 95% CI across participants. Asterisks identify days on which significant differences were observed among the modulation groups and serial positions during the follow-up two-sided independent-sample t-tests. These indicate significantly higher recall probability within the primacy cluster in the DLPFC group, relative to the sham group, in Experiment 1, on day 2 (t38 = 2.075, P = 0.045, d = 0.66), day 3 (t38 = 3.660, P = 0.001, d = 1.16), day 4 (t38 = 3.381, P = 0.002, d = 1.07) and 1 month (t38 = 2.381, P = 0.022, d = 0.75) timepoints and significantly higher recall probability within the recency cluster in the IPL theta group, relative to the sham group, in Experiment 1, on day 3 (t38 = 2.631, P = 0.012, d = 0.83), day 4 (t38 = 4.650, P = 3.9 × 10−5, d = 1.47) and 1 month (t38 = 2.253, P = 0.030, d = 0.98) timepoints. b, Mean recall probabilities as in a for Experiment 2 groups: sham (top, grays, n = 20), DLPFC theta (middle, blues, n = 20) and IPL gamma (bottom, oranges, n = 20). No significant differences in mean recall probabilities were observed in Experiment 2. Comparisons within the primacy and recency cluster were hypothesis driven and were not subjected to any corrections for multiple comparisons. Comparisons within the middle position clusters were exploratory and subjected to Bonferroni correction. *P < 0.05, **P < 0.01 and ***P < 0.001. CI, confidence interval; NS, not significant.
IPL theta modulation selectively improves WM
We also examined a day × serial position × group interaction effect between sham and IPL theta groups in Experiment 1, using a mixed ANOVA. This interaction effect was significant (F9.0,342.9 = 3.111, P = 0.001, ηp2 = 0.076). Follow-up mixed ANOVAs demonstrated the specific days at which the serial position × group interaction was significant. Improvements in memory performance were observed on day 3 (F3.6,137.3 = 5.713, P < 0.001, ηp2 = 0.131), day 4 (F3.1,120.6 = 18.93, P < 0.001, ηp2 = 0.333) and 1 month after intervention (F2.8,109.3 = 3.852, P = 0.013, ηp2 = 0.092). Additional ANOVAs revealed that the day × group interaction was significant only for the recency serial position cluster (F2.6,100.7 = 5.116, P = 0.004, ηp2 = 0.119) but not other position clusters (Fs < 1.005, ps > 0.407). Independent-sample t-tests revealed significant improvements in the recency effect in the IPL theta group relative to sham group on day 3 and day 4 of neuromodulation, and these improvements were sustained at the 1-month post-intervention timepoint (Fig. 2a, top and bottom). The pattern of effects was not affected by inclusion of additional covariates (Supplementary Tables 1–3). The results suggest that theta-rate neuromodulation aimed at left IPL selectively enhanced WM in older individuals without behavioral costs to other memory systems. These selective memory improvements were evident by day 3 of the intervention and lasted for at least 1 month, relative to memory performance of participants in the sham group.
Specific location and frequency combinations are necessary
Experiment 1 demonstrated improved WM function with repetitive modulation of IPL theta rhythms. However, both theta and gamma frequency rhythms contribute to WM function32. As a result, it is important to confirm whether WM improvements occur specifically due to theta modulation in the IPL or whether they are also possible with gamma modulation in the IPL. Likewise, it is important to confirm whether LTM improvements with DLPFC modulation are specifically due to gamma entrainment or whether theta entrainment can produce similar effects. To test these possibilities, we performed Experiment 2 following the same design as Experiment 1, except that the three experimental groups received sham, IPL gamma or DLPFC theta modulation. A mixed ANOVA with day (baseline, day 1, day 2, day 3, day 4 and 1 month) and serial position (primacy, middle 1, middle 2, middle 3 and recency) as within-subjects factors and group (sham, DLPFC theta and IPL gamma) as between-subjects factor failed to find any significant differences in the recall performance (day × serial position × group: F25.3,721.9 = 0.535, P = 0.971, ηp2 = 0.018; Fig. 2b). This was not influenced by inclusion of covariates (F24.3,633.2 = 0.630, P = 0.916, ηp2 = 0.024). This indicates that the improvements we observed in Experiment 1 are both location specific and frequency specific: modulation of theta rhythms in the IPL, and not gamma rhythms, improved WM without affecting LTM; and modulation of gamma rhythms in the DLPFC, and not theta rhythms, improved LTM without affecting WM. Moreover, the two different frequency conditions for a given brain region across the two experiments serve as active controls for each other. Consequently, these findings confirm that the effects observed in Experiment 1 are not due to any non-specific effect of tACS such as transretinal or transcutaneous modulation33 but due to frequency-specific entrainment of relevant brain circuits.
Validation of sham and pre-intervention baseline controls
To test the validity of the control procedures and, thus, the strength of the principal findings, we examined the recall performance at the pre-intervention baseline timepoint across groups (Experiment 1: sham, DLPFC gamma and IPL theta; Experiment 2: sham, DLPFC theta and IPL gamma; Fig. 2a,b, ‘Baseline’ timepoint) and serial positions. A mixed ANOVA comparing these groups did not find a significant interaction effect of serial position (primacy, middle 1, middle 2, middle 3 and recency) or group (Experiment 1: sham, DLPFC gamma and IPL theta; Experiment 2: sham, DLPFC theta and IPL gamma) on performance at the pre-intervention baseline timepoint with or without covariates in either experiment (Fs < 0.925, ps > 0.488). These results suggest that the three groups in each experiment did not differ in their baseline memory performance for any serial position cluster. Thus, the selective effects of neuromodulation on serial positions were not driven by any inherent differences within the three groups in either experiment. Furthermore, we tested how stable and reliable the recall performance was for serial position clusters within the sham group across timepoints in each experiment (baseline, day 1, day 2, day 3, day 4 and 1 month; Fig. 2a,b, top). A repeated-measures ANOVA examining the day × serial position interaction effect within the sham group did not show any significant differences with or without covariates in either experiment (Fs < 1.603, ps > 0.135). Together, these results demonstrate the stability and reliability of memory performance during the pre-intervention baseline across different groups of participants and within the same group of participants over different timepoints of assessment lasting more than 1 month, which together strengthen confidence in the validity of the control procedures and the resulting tACS improvements.
Four-day improvement rate predicts benefits 1 month later
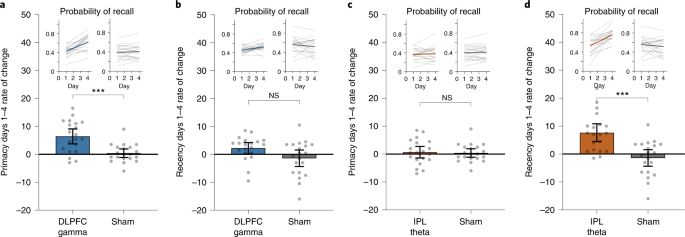
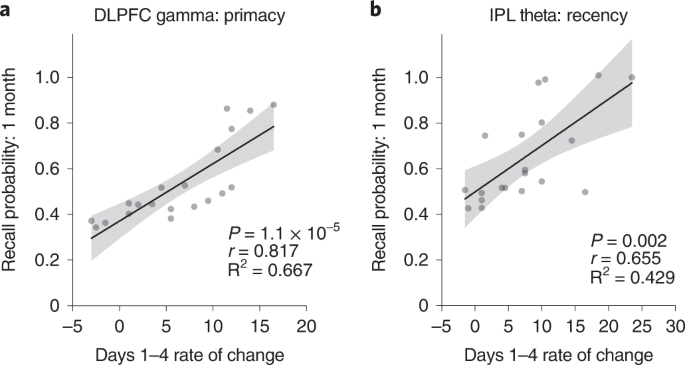
Having established the location specificity and frequency specificity of the memory improvements, we next explored factors that predict sustainable effects. We evaluated the rates of improvement in LTM (primacy) and WM (recency) over the 4-day intervention in Experiment 1. Of the 20 participants in the DLPFC gamma group, 17 (85%) showed a positive rate of primacy improvements over the 4 days. Similarly, of the 20 participants receiving IPL theta modulation, 18 (90%) showed a positive rate of recency improvements over the 4 days. By modeling these data using linear regression, we observed a significantly higher mean rate of improvement for primacy over 4 days of DLPFC modulation relative to sham and for recency during IPL modulation relative to sham (Fig. 3), but the reverse was not true. Neither recency in the DLPFC gamma group nor primacy in the IPL theta group were significantly different relative to sham after Bonferroni correction (Fig. 3). Strikingly, the rate of improvement over the course of the intervention was highly predictive of post-intervention memory benefits: participants with greater primacy improvement rates during DLPFC modulation showed the largest primacy benefits at 1 month (r18 = 0.817, Pcorr < 0.001), and participants with greater recency improvement rates during IPL modulation showed the largest recency benefits at 1 month (r18 = 0.655, Pcorr = 0.002) (Fig. 4a,b). Again, the opposite was not true (DLPFC recency: r18 = 0.243, Pcorr = 0.303; IPL primacy: r18 = 0.385, Pcorr = 0.094; Pearson test, two-sided, Bonferroni correction, Pcorr < 0.0125). The results indicate that not only did the overwhelming majority of older individuals experience memory improvements—selectively for WM or LTM depending on the nature of neuromodulation—the size and, thus, the sustainability of the memory improvements 1 month later were highly predicted by the speed of memory improvements during the 4-day intervention.
Regression analyses were performed to test for the presence of a linear relationship across participants between the rate of change in recall performance during neuromodulation and the recall performance 1 month after the intervention. a, Scatter plot shows the speed (rate of change) of each participant’s improvement in primacy over 4 days of DLPFC gamma neuromodulation against the same individual’s primacy score 1 month after intervention in Experiment 1. Gray dots show individual participant data (n = 20). The solid line indicates a regression fit, and the error bands show 95% CI. This exploratory analysis identified significant, positive linear relationships between the rate of primacy improvements and 1-month primacy performance in the DLPFC gamma group (r18 = 0.817, P = 1.1 × 10−5). b, Scatter plot as in a for recency in the IPL theta group (n = 20) in Experiment 1. Significant, positive, linear relationship was observed between the rate of recency improvements and 1-month recency performance in the IPL theta group (r18 = 0.655, P = 0.002). These analyses were subjected to Bonferroni correction for multiple comparisons (Pcorr < 0.0125). CI, confidence interval.
General cognitive function moderates memory improvements
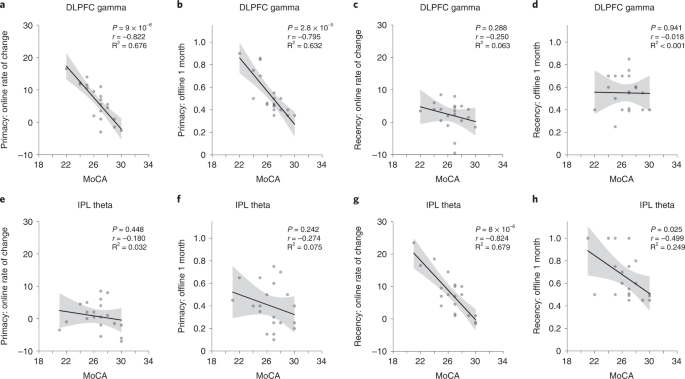
Previous studies demonstrated that the effects of tACS can be modulated by baseline behavioral34 and neural35 states. We, therefore, examined whether memory improvements due to neuromodulation in Experiment 1 were moderated by levels of baseline cognitive function. We performed participant-wise regression of MoCA scores, memory performance at the 1-month post-intervention timepoint and the rate of change in memory performance during days 1–4 for the primacy and recency serial position clusters (Fig. 5). Participants with lower baseline cognitive performance in the DLPFC gamma group showed higher rates of primacy improvement over the 4-day intervention (r18 = −0.822, P < 0.001; Fig. 5a) and showed larger primacy gains at 1 month after intervention (r18 = −0.795, P < 0.001; Fig. 5b). No such relationships held for recency in the DLPFC gamma group (rs18 > −0.25, ps > 0.288; Fig. 5c,d). Moreover, participants with lower baseline cognitive performance in the IPL theta group showed higher recency improvement rates over the 4-day period (r18 = −0.824, P < 0.001; Fig. 5g) and greater recency improvements after 1 month (r18 = −0.499, P = 0.025; Fig. 5h). Consistent with previous analyses, the level of cognitive performance did not predict changes in primacy during or after IPL modulation (rs18 > −0.274, ps > 0.242; Fig. 5e,f). Thus, older participants with relatively low baseline cognition more strongly revealed the preferential nature of the gamma-rate DLPFC and theta-rate IPL modulation effects on primacy and recency, respectively. This conclusion, which suggests distinctive functions of prefrontal gamma rhythms for LTM and parietal theta rhythms for WM, was reinforced by the absence of participant-wise correlations in the sham group between baseline cognitive behavior and primacy or recency measured during or after sham (rs18 > 0.064, ps > 0.79). These results suggest that the large-scale population dynamics that support memory function in older people can be differentially modulated depending on the individual level of general cognitive performance.
Participant-wise correlations between general cognitive function, quantified by MoCA scores and memory performance measures in the DLPFC gamma (n = 20) and IPL theta (n = 20) groups. Memory performance measures include ‘online’ measures quantified by the rate of change in memory performance across days 1–4 of neuromodulation and ‘offline’ measures quantified by the memory performance at the 1-month post-intervention timepoint, separately computed for the primacy and recency clusters. a, Correlation between MoCA scores and online measure for the primacy cluster in the DLPFC gamma group (r18 = −0.822, P = 9 × 10−6). b, Correlation between MoCA scores and offline measure for the primacy cluster in the DLPFC gamma group (r18 = −0.795, P = 2.8 × 10−5). c, Correlation between MoCA scores and online measure for the recency cluster in the DLPFC gamma group (r18 = −0.250, P = 0.288). d, Correlation between MoCA scores and offline measure for the recency cluster in the DLPFC gamma group (r18 = −0.018, P = 0.941). e, Correlation between MoCA scores and online measure for the primacy cluster in the IPL theta group (r18 = −0.180, P = 0.448). f, Correlation between MoCA scores and offline measure for the primacy cluster in the IPL theta group (r18 = −0.274, P = 0.242). g, Correlation between MoCA scores and online measure for the recency cluster in the IPL theta group (r18 = −0.824, P = 8 × 10−6). h, Correlation between MoCA scores and offline measure for the recency cluster in the IPL theta group (r18 = −0.499, P = 0.025). Solid lines indicate the regression fit across participants between the MoCA scores and the neuromodulation effects (rate of change during modulation/recall probability after 1 month) in the primacy or recency clusters. Error bands show 95% CI. These hypothesis-driven analyses were not subjected to multiple comparisons correction. CI, confidence interval.
Replication of primary findings in an independent sample
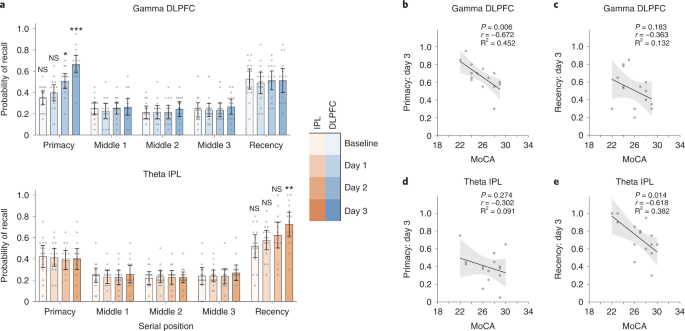
We performed an additional experiment to test whether the primary observations from Experiment 1 replicate in an independent sample. Experiment 3 consisted of 30 older participants randomized to receive either DLPFC gamma or IPL theta neuromodulation during performance of the free recall task. The neuromodulation protocol followed was largely similar to Experiment 1, except that the neuromodulation was performed for three rather than four consecutive days and did not include a long-term follow-up. Memory performance was examined at baseline and during each neuromodulation session. A mixed ANOVA with day (baseline, day 1, day 2 and day 3) and serial position (primacy, middle 1, middle 2, middle 3 and recency) as within-subjects factors and group (DLPFC gamma and IPL theta) as between-subjects factor revealed significant differences in memory performance (day × serial position × group: F7.9,220.8 = 6.315, P < 0.001, ηp2 = 0.184; Fig. 6a), and this effect remained significant even after accounting for covariates (F7.7,176.1 = 5.887, P < 0.001, ηp2 = 0.204). Follow-up ANOVAs revealed a significant interaction between serial position and group on days 2 and 3 of neuromodulation and a significant interaction between day and group for the primacy and recency clusters (Supplementary Table 4). Two-sided independent-sample t-tests showed that memory performance in the primacy cluster was significantly improved in the DLPFC gamma group relative to the IPL theta group on day 2 and day 3 of neuromodulation (Fig. 6a, top). Performance in the recency cluster was significantly higher in the IPL theta group relative to the DLPFC gamma group on day 3 of the intervention (Fig. 6a, bottom). These results parallel observations from Experiment 1 (Fig. 2a, left). Baseline performance did not differ between the two groups (Supplementary Table 4), thus ruling out non-specific between-group differences. Examining the relationship between baseline cognitive function and memory performance, we found that individuals with lower MoCA scores in the DLPFC gamma group showed better memory performance at day 3 only in the primacy cluster (r13 = −0.672, P = 0.006; Fig. 6b,c), whereas those with lower MoCA scores in the IPL theta group showed better memory performance on day 3 only in the recency cluster (r13 = −0.618, P = 0.014; Fig. 6d,e), similar to the findings in Experiment 1 (Fig. 5). Together, these observations in an independent sample of participants replicate the primary findings of Experiment 1, further strengthening confidence in the inferences drawn from them.
a, Mean recall probabilities plotted across serial position clusters on all measurement days for Experiment 3 groups: DLPFC gamma (top, blues, n = 15) and IPL theta (bottom, oranges, n = 15). Gray dots show individual participant data. Mean of center shows the average recall probability, and the error bars show 95% CI across participants. Following mixed ANOVAs (see text), two-sided independent-sample t-tests identified significant differences in recall probability across days, groups and serial positions (see asterisks). Participants in the DLPFC gamma group showed higher recall probability within the primacy cluster on day 2 (t28 = 2.2, P = 0.037, d = 0.80) and day 3 (t28 = 4.467, P = 1.25 × 10−4, d = 1.63). Participants in the IPL theta group showed higher recall probability within the recency cluster on day 3 (t28 = −2.868, P = 0.008, d = 1.05). Comparisons within the primacy and recency cluster were hypothesis driven and were not subjected to any corrections for multiple comparisons. Comparisons within the middle position clusters were exploratory and subjected to Bonferroni correction. *P < 0.05, **P < 0.01 and ***P < 0.001. NS, not significant. b, Participant-wise correlations between MoCA scores and memory performance in the primacy cluster on day 3 of neuromodulation in the DLPFC gamma group (r13 = −0.672, P = 0.006). Similar correlations are shown for the recency cluster performance on day 3 in the DLPFC gamma group (r13 = −0.363, P = 0.183) in c, for the primacy cluster performance in the IPL theta group (r13 = −0.302, P = 0.274) in d and for the recency cluster performance in the IPL theta group (r13 = −0.618, P = 0.014) in e. Gray dots indicate individual participant data. Solid line indicates a regression fit, and the error bands show 95% CI across participants. These hypothesis-driven regression analyses were not subjected to multiple comparisons correction. CI, confidence interval.
Discussion
We present evidence for selective improvements in WM and LTM in older adults through dissociable spatiospectral entrainment of brain rhythms, and the improvements are sustained for at least 1 month after intervention. Experiment 1 showed that selective changes to WM and LTM function are possible through entrainment of theta rhythms in the IPL and gamma rhythms in the DLPFC, respectively. Experiment 2 showed that switching the modulation frequencies between the two regions did not produce any benefits. Consequently, it is the combination of anatomical location and rhythmic frequency that determines the appropriate substrate for memory improvement. Moreover, it confirmed that the improvements observed during Experiment 1 were due to entrainment of functionally specific brain circuits and not due to non-specific effects such as transretinal or transcutaneous stimulation33. In addition, we observed greater improvements in individuals with poorer cognitive function. These findings were further replicated in an independent sample in Experiment 3. We further found that the speed with which the memory function improves during the intervention predicts memory strength 1 month after the intervention, thus yielding an important metric to measure treatment responsiveness in future studies. Together, these findings suggest that memory function can be selectively and sustainably improved in older adults through modulation of functionally specific brain rhythms.
The specificity with which distinct rhythmic neuromodulation protocols affected different memory functions may seem surprising given the literature documenting general involvement of both frontal and parietal regions and both theta and gamma rhythms to WM and LTM function36,37. This is particularly the case because neuromodulation was performed during both encoding and recall of all words presented during a list. Our findings strongly suggest that our interventions manipulated two distinct cognitive operations. Following the dual-store framework, we hypothesize that IPL theta modulation improved WM operations. However, unlike in previous neuromodulation studies with visuospatial memoranda18, we do not think that IPL theta modulation improved WM capacity per se. If that were the case, then improvements in memory performance would have also been observed in some middle position clusters in addition to the recency cluster. We also do not expect increases in general attention function with IPL theta modulation. Although parietal theta rhythms are hypothesized to facilitate attentional sampling38, there is little evidence to suggest changes in attention with parietal theta entrainment39. Instead, we propose that IPL theta modulation may have facilitated the temporal segregation between successive memory representations, minimizing interference among them15. Moreover, theta rhythms are also known to facilitate temporal context-mediated recall40, potentially reflecting a common neurophysiological mechanism underlying preserved maintenance and context-based retrieval of WM representations. Intrinsic limitations on the WM capacity, unaffected by neuromodulation, may constrain these improvements to only the later words in the list, thereby only improving the recency cluster. If so, then these findings may reflect an additional approach for non-invasively improving WM function within the influential theta–gamma cross-frequency coupling theory15, besides changing memory capacity18. The possibility that, although IPL theta modulation may have facilitated maintenance and recall of later list items, it may not have improved the transfer of previously presented information to LTM, may have further contributed to the selectivity of effects. This could be due to the presence of distinct encoding mechanisms for the two memory stores, a possibility supported by a recent transcranial magnetic stimulation (TMS) study14. Alternatively, transfer of representations between the two memory systems may involve separate executive control processes41 that were unaffected by the current neuromodulation design. Consequently, IPL theta modulation may not have affected memory representations in the primacy cluster. Instead, improvements in the primacy effect emerged selectively with DLPFC gamma modulation. This protocol may have selectively improved the ability to retrieve the representations separately encoded or transferred to LTM, by potentially affecting hippocampus and other temporal lobe structures42, which also simultaneously exhibit gamma activity during delayed recall36. A previous neuromodulation study, although examining memory function in young adults with single-session conventional tACS, aligns with this proposal22. Thus, although both theta and gamma rhythms, and both DLPFC and IPL regions, are known to generally contribute to WM and LTM performance, they may index distinct cognitive processes that selectively underlie the dissociable improvements observed in the current study.
The findings of the present study also contribute to the debate surrounding theoretical models of free recall. Segregated neural bases of primacy and recency effects have been a hotly debated topic in neuropsychology with conflicting evidence14,43. The selective modulation of primacy and recency effects observed in the current study support distinct underlying mechanisms, in agreement with the dual-store models11 and neuropsychological observations12,13. However, our findings, at present, are not incompatible with alternative models of free recall. For instance, one theory attributes primacy effects to ‘long-term working memory’ in which long-term storage and retrieval operations support WM function contingent upon expertise-dependent retrieval structures44,45. This view is not inconsistent with the aforementioned hypothesis that DLPFC gamma neuromodulation may have affected retrieval from LTM, albeit— in this view—in service of WM. A way to disambiguate between these two perspectives is to use the method of personalization to modulate expertise45, in which case this theory would predict a stronger effect of DLPFC gamma neuromodulation in the presence of stronger expertise-dependent retrieval structures. Furthermore, although the contextual retrieval theories are not designed to explain primacy effects, deficits in primacy effects in older adults have been attributed to attentional processes46, which, in turn, are associated with DLPFC gamma activity47. It is possible that DLPFC gamma neuromodulation may have further enhanced the intrinsic gradient in the efficiency of encoding mechanisms with benefits to early events in a series46. Notably, increased gamma activity in the temporal lobe is associated with this effect17. As discussed above, DLPFC gamma neuromodulation may have led to downstream effects on gamma activity in the temporal lobe structures42, enhancing the primacy effect. Whether DLPFC gamma neuromodulation specifically affects LTM retrieval processes or attentional mechanisms can be potentially addressed through a granular analysis of memory performance within the primacy cluster. For instance, the LTM retrieval account predicts an additive shift to memory performance with increasing serial position in the primacy cluster due to similar benefits to retrieval processes at all serial positions, whereas the attentional account predicts a reduction in the slope of memory performance as a function of the serial position, thereby reflecting a stabilization in sustained attention46. The success of the neuromodulation protocol in selectively manipulating the primacy effect will be a powerful tool to test these competing predictions. Future studies that are sufficiently powered to systematically test these hypotheses can disambiguate between these competing predictions to refine and reconcile the various theories of free recall.
This work contributes to the growing literature that suggests potential clinical benefits for memory function in older adults with non-invasive techniques7. The protocols used in the current study demonstrate that memory function can be selectively improved for at least 1 month after a 4-day intervention. These long-lasting effects may arise due to neuroplastic changes48 after phase-locking of intrinsic brain rhythms with tACS49. In addition, these findings suggest that functional differentiation, which typically reduces with aging50, can be promoted through functionally specific neuromodulation. Findings from the present study may motivate several lines of investigation to further examine their clinical potential. For instance, future studies should examine the generalizability of these findings to different cognitive paradigms spanning memory function across various sensory domains and replicate them in larger study samples. Moreover, how to promote sustainable effects that go beyond the 1-month duration observed in the current study needs to be determined. Personalization of the neuromodulation protocol according to individual anatomical and functional characteristics is one possible approach6. In addition, the specific frequency within the theta and gamma ranges, the number and duration of modulation sessions, the optimal gap between successive sessions and the interaction of baseline cognitive and neural function with these metrics can be systematically varied to determine the most optimal modulation designs. Furthermore, in addition to MoCA, future studies should use more comprehensive neuropsychological assessments to quantify baseline cognitive function and its association with tACS-induced improvements. Finally, beyond potential benefits to healthy older adults, the translational implications for people with neuropsychiatric and neurodegenerative disorders, particularly those with selective memory deficits10 and at risk for dementia5, should be examined. Findings from the present study serve as a stepping stone toward investigating these questions of clinical interest.
More at link.
No comments:
Post a Comment