But why go thru all the trouble of stem
cells if exosomes are the reason for the benefits? Which must be why no
one seems to be monitoring stem cell survival.
Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations
Induced Pluripotent Stem Cells for Ischemic Stroke Treatment
The latest here:
Immune response treated with bone marrow mesenchymal stromal cells after stroke
- 1Department of Neurosurgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 2School of Clinical Medicine, Guizhou Medical University, Guiyang, China
Stroke is a leading cause of death and long-term disability worldwide. Tissue plasminogen activator (tPA) is an effective treatment for ischemic stroke. However, only a small part of patients could benefit from it. Therefore, finding a new treatment is necessary. Bone marrow mesenchymal stromal cells (BMSCs) provide a novel strategy for stroke patients. Now, many patients take stem cells to treat stroke. However, the researches of the precise inflammatory mechanism of cell replacement treatment are still rare. In this review, we summarize the immune response of BMSCs treated to stroke and may provide a new perspective for stem cell therapy.
Introduction
Stroke is a leading cause of death and long-term disability worldwide (1). Every year ~15 million people suffer from stroke in the world (2). Immune response plays a key factor in stroke progression. Neuroinflammation is an inflammatory response within the central nervous system (CNS), involving many different mediators such as cytokines, chemokines, reactive oxygen species and secondary messengers (3). Oxygen and glucose deprivation following brain tissue damage results in necrosis of neurons and released the different damage-associated molecular patterns (DAMP) which trigger neuroinflammation (4). DAMP include a wide variety of endogenous molecules released on tissue injury, which alter the blood-brain barrier (BBB) permeability, promote peripheral immune cell infiltration, and accelerate tissue edema and brain injury (5). Then microglia are activated and polarize M1 and M2 phenotypes. M1 microglia upregulate a variety of pro-inflammatory mediators which continually damage BBB integrity (6). In the periphery, spleen plays a pivotal role in humoral immunity. Following compromised BBB, spleen releases a mass of peripheral immune cells and inflammatory cytokines infiltrating brain insult. Those different pathways collectively exacerbate the secondary progression of ischemic brain injury (7). We summarize the inflammatory mechanism after stroke (Figure 1).
By now tissue plasminogen activator (tPA) is a proven treatment for acute ischemic stroke (8). However, the use of tPA is restricted by the narrow time window of 4.5 h after ischemic stroke onset, which has limited its use to only a small minority of patients (9). Thrombectomy also is an available approval therapy, which has restricted therapeutic outcome (10). Hence finding a novel effective treatment that could ameliorate the secondary progression of ischemic stroke injury will benefit stroke patients who cannot use tPA (11).
Bone marrow mesenchymal stromal cells (BMSCs) offer an innovative strategy. Stem cell is a kind of special cell which could self-renew, proliferate, and differentiate into specialized cells for cell replacement treatment to stroke (12). Many researches showed that transplanted BMSCs home to sites of injury, which may depend on chemotactic signals (13). Zheng et al. observed that intravenously delivered BMSCs are entrapped in lung microvasculature and are cleared to the liver in 1 day (14). Other researches demonstrated that injected BMSCs preferentially migrate to spleen after stroke (11). Cells through intracerebral transplantation could directly migrate into the infract brain tissue, however, it is more invasive (15). BMSCs take effects through different pathways after stroke, including migrating into ischemic infarction (11), proliferating neuroblasts, replacing impaired cells (16), promoting angiogenesis and neurogenesis (17) and secret a great bunch of neurotrophins. However, BMSCs also cause thrombus and increased intracranial hypertension (15). From many recent researches, except the effects mentioned above, BMSCs could mediate neuroinflammation to accelerate neurofunctional recovery. Therefore, the present review teases out the immunomodulatory effects of BMSCs transplantation after stroke.
BMSC and central nervous system
With the release of DAMPs following stroke, the microglia become activated, polarizing M1 and M2 phenotypes (18). M1 microglia secrete pro-inflammatory mediators, such as IL-1, IL-6, IL-12, TNF-α, and aggravate brain damage. In contrast, M2 microglia secrete anti-inflammatory cytokines, such as TGF-β and IL-10, accelerating neural repair (19). Stromal derived factor-1 (SDF-1) is mainly produced in microglia/macrophage in a rat middle cerebral artery occlusion (MCAO) model. Shiota et al. found a mesenchymal stem cell (MSC) line (B10) transplantation increased SDF-1 mRNA level from an early time point that persisted until 14 days after MCAO (20). Some researchers found that transplanted BMSCs reduced microglia activation, conferring immunomodulatory effect (21, 22). A study by Nijboer et al. indicated that the number of M2-like (CD206+) microglia was highly increased through intranasal MSC administration (23). In another article, Yang et al. confirmed those findings that BMSCs transplantation promoted M2 phenotype polarization, and decreased the expression of M1 maker in vivo and in vitro (24). Those researches suggest that BMSCs transplantation could impact M2 polarization meditating inflammatory response.
Astrocytes maintain structure for neurons and contribute to keeping homeostasis of the extracellular environment (25). Also, activated astrocytes play a key participant in neuroinflammation by secreting a large number of inflammatory mediators. The activation of astrocytes could result in dense glial scars, exacerbating neurological deterioration and affecting long-term neuronal recovery (26). Shiota et al. also found B10 transplantation increased the differentiation of neuronal progenitor cells to astrocytes (20). A group of researchers found that BMSCs co-culture enhanced the resistance of astrocytes to hemin neurotoxicity. And they found that BMSCs transplantation promotes astrocytes vimentin expression, and enhance astrocytes antioxidation (26). Zhang et al. co-cultured BMSCs with neurons and astrocytes which exposed to oxygen-glucose deprivation, and found that BMSCs exerted neuroprotection through hindering the apoptosis of neurons and astrocytes (27). Those evidences showed that BMSCs diminished the apoptosis of astrocytes and enhanced its neuroprotection.
Oligodendrocyte precursor cells (OPCs) are immature forms of oligodendrocytes which are essential for repair of damaged white matter after ischemic injury (28). After brain ischemia, immature oligodendrocytes proliferate in the peri-infract areas. Then newly created oligodendrocytes establish contact with un-myelinated axons and form functional myelin sheaths around them (29). BMSCs could reduce the expression of IL-1β protein that could impede the recruitment of OPCs (30). It's reported that BMSCs treatment increased oligodendrogenesis after MCAO, and elevated the number of Nissl-stained neurons in the cortex. Hence, researchers indicated that BMSCs transplantation protects the myelin sheath and promotes axonal restoration (31). In the study by Zarriello et al., OPCs co-cultured with BMSCs increased myelination compared to control group (32). There are some reports that M2 phenotype microglia promoted OPCs differentiation (33). It suggested that BMSCs facilitated OPCs differentiation through promoting M2 phenotype polarization and improved myelination.(Interesting)
 Zili Wang
Zili Wang Xudong Wang1,2†,
Xudong Wang1,2†,  Guangtang Chen
Guangtang Chen Kaya Xu
Kaya Xu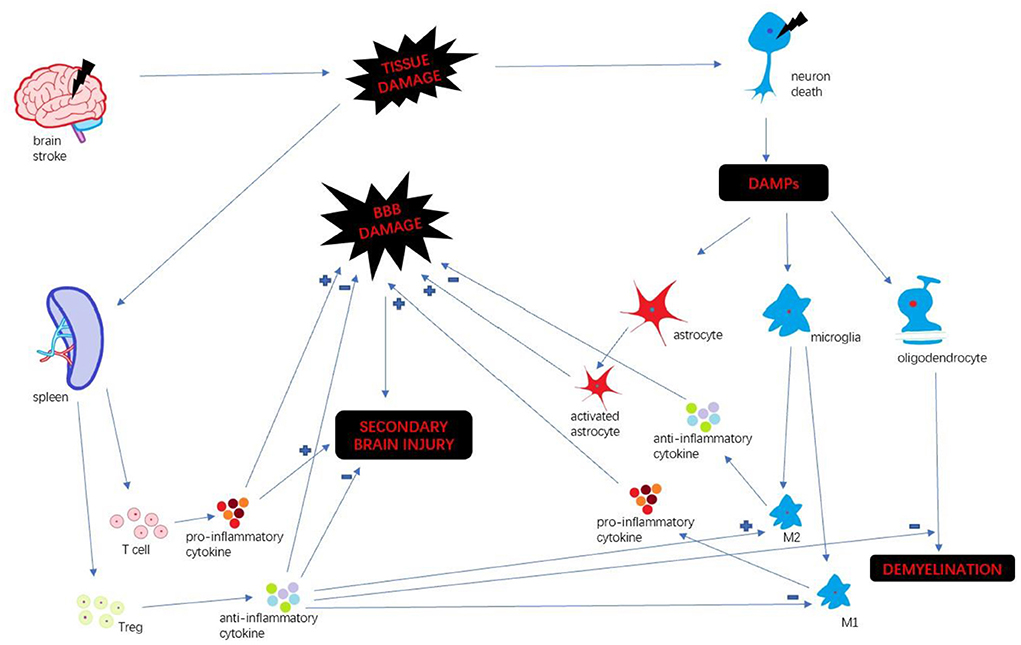
No comments:
Post a Comment