No clue how quantifying any of this helps survivors recover. I don't understand how mentors and senior researchers can allow such useless research. The actual goal of stroke research is survivor recovery, if you can't link your research to that in some way it was useless.
System identification: a feasible, reliable and valid way to quantify upper limb motor impairments
Journal of NeuroEngineering and Rehabilitation volume 20, Article number: 67 (2023)
Abstract
Background
Upper limb impairments in a hemiparetic arm are clinically quantified by well-established clinical scales, known to suffer poor validity, reliability, and sensitivity. Alternatively, robotics can assess motor impairments by characterizing joint dynamics through system identification. In this study, we establish the merits of quantifying abnormal synergy, spasticity, and changes in joint viscoelasticity using system identification, evaluating (1) feasibility and quality of parametric estimates, (2) test–retest reliability, (3) differences between healthy controls and patients with upper limb impairments, and (4) construct validity.
Methods
Forty-five healthy controls, twenty-nine stroke patients, and twenty cerebral palsy patients participated. Participants were seated with the affected arm immobilized in the Shoulder-Elbow-Perturbator (SEP). The SEP is a one-degree-of-freedom perturbator that enables applying torque perturbations to the elbow while providing varying amounts of weight support to the human arm. Participants performed either a ‘do not intervene’ or a resist task. Elbow joint admittance was quantified and used to extract elbow viscosity and stiffness. Fifty-four of the participants performed two sessions to establish the test–retest reliability of the parameters. Construct validity was assessed by correlating system identification parameters to parameters extracted using a SEP protocol that objectifies current clinical scales (Re-Arm protocol).
Results
Feasibility was confirmed by all participants successfully completing the study protocol within ~ 25 min without reporting pain or burden. The parametric estimates were good with a variance-accounted-for of ~ 80%. A fair to excellent test–retest reliability was found (
) correlation with parameters from the Re-Arm protocol.
Conclusions
This work demonstrates that system identification is feasible and reliable for quantifying upper limb motor impairments. Validity was confirmed by differences between patients and controls and correlations with other measurements, but further work is required to optimize the experimental protocol and establish clinical value.
Introduction
Upper limb motor impairments in hemiparetic limbs such as after stroke and cerebral palsy (CP) are currently assessed by a set of well-established clinical scales aiming to quantify, among others, muscle weakness, abnormal synergy, spasticity and changes in joint viscoelasticity [1]. These include tests like the Brunnstrom Fugl-Meyer, Modified Ashworth Scale (MAS), and Modified Tardieu Scale (MTS). The motor impairments identified using these tests assist in selecting a treatment approach and are used to monitor improvement. However, the tests have several known limitations, which include their poor validity, reliability, and sensitivity [2,3,4,5,6]. Whereas the need for objective and reliable assessment of motor impairments is widely recognized [7, 8], no tests have yet successfully replaced traditional clinical scales.
Robotics can objectively quantify the motor impairments of muscle weakness, abnormal synergy, spasticity, and changes in joint viscoelasticity [9,10,11]. Recently, a robotic device was developed and validated to assess these four impairments using one single device (the Shoulder-Elbow-Perturbator—SEP) [12]. By using the SEP to mimic current clinical tests, an earlier finding was confirmed that upper limb synergy can be quantified by the change in elbow range of motion (ROM) when gradually reducing arm weight support [13]. Likewise, spasticity and viscoelasticity can be objectively quantified by imposing passive joint movements at low and high speeds [9, 14, 15]. The robotics used in the aforementioned studies leveraged the accurate recording of joint angles and torques to provide a reliable way to quantify motor impairments during passive and active joint movements.
Motor impairments can also be quantified with the help of robotics by applying external mechanical perturbations while participants perform either a ‘do not intervene’ (DNI) or resist task [16, 17]. These tasks imply the participant is fully relaxed (DNI task) or attempts to maintain a constant joint angle or torque (resist task) while the joint is rotated within a small ROM (e.g., root mean square rotation of 0.5–1°). Recordings of the joint angle and joint torque are subsequently scrutinized using system identification techniques to characterize the joint dynamics. System identification enables us to quantify both the intrinsic (non-neural) and reflexive (neural) contribution to the joint dynamics [18, 19], captured in the joint’s inertia, viscosity, and stiffness parameters. These parameters can be translated to clinically described phenomena such as synergy (i.e., aberrant elbow stiffness when lifting the arm), spasticity (i.e., enhanced reflexive activity), or changes in viscoelasticity (i.e., increased intrinsic joint viscosity and stiffness).
In this study, we aim to establish the merits of quantifying abnormal synergy, spasticity, and changes in joint viscoelasticity using system identification. This is achieved by (1) confirming the feasibility to perform a system identification protocol and quality of parametric estimates, (2) establishing the test–retest reliability for the system identification parameters, (3) comparing obtained parameters between healthy controls and patients with upper limb impairments and (4) test construct validity by comparing the system identification parameters for each motor impairment with parameters obtained using four tests as part of a recently proposed robotic assessment protocol (Re-Arm) [12].
Methods
Participants
We recruited adult patients from the outpatient clinic of the Rijndam Rehabilitation Center that presented upper limb motor impairments due to stroke and CP. Patients were only included if they: (1) had a clinically-confirmed upper limb impairment, (2) were able to achieve active shoulder abduction (up to 80°), (3) were able to achieve visible active elbow extension, and (4) had a minimal passive range-of-movement for shoulder abduction of 80° and horizontal shoulder adduction of 45°. Exclusion criteria were: (1) hemiplegic shoulder pain, (2) a history of pre-existing neuromuscular disorders affecting upper limb function, (3) fixed upper limb contractures, and (4) the inability to understand verbal instructions. We only included stroke patients when they were considered chronic, suffering the stroke at least six months before study inclusion.
For comparison, we recruited a group of age-matched healthy controls without a known history of neurological or orthopedic disorders. The study was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, (protocol number NL64660.078.18), and conducted following the declaration of Helsinki.
Experimental setup
Shoulder elbow perturbator
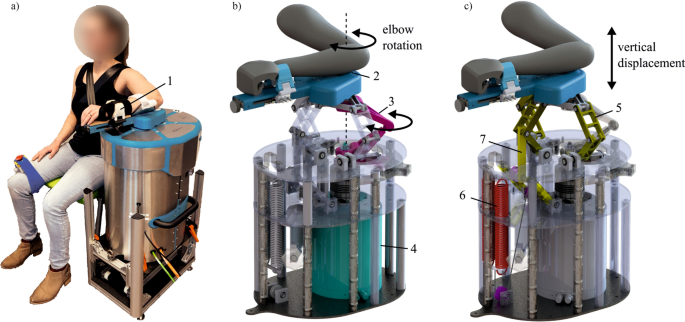
The Shoulder-Elbow-Perturbator (SEP—Hankamp Rehab, Enschede, The Netherlands) was used to assess participants' elbow dynamics. The design of the SEP enables independent manipulation of elbow angle and weight support of the human arm [12]. A direct-drive servo motor (HIWIN TMS3C, Offenburg, Germany) attached to a lever supporting the lower arm controlled the elbow angle by aligning its axis to the medial epicondyle of the humerus (Fig. 1). A computer with Etherlab and MATLAB Simulink was used to control the SEP and capture elbow torque and angle with a sample rate of 1 kHz.
The Shoulder Elbow Perturbator (SEP), a robotic device to quantify multiple upper limb impairments. a Participant positioned in the SEP with the shoulder abducted in 80°, the forearm strapped (1) to the lever arm of the SEP, and the medial epicondyle of the humerus aligned with the motor rotation axis. b Internals of the SEP showing that the motor (4) transmits a torque through the torque link (3), allowing elbow rotation (2). c Internals of the SEP showing the shoulder abductor manipulation mechanism. The sarrus linkages (5) allow vertical displacement of the arm, and the arm is supported by two springs (6) with an upward force. With the cable routing and pulley configuration, the upward force can be manipulated independently of the linkage position (7). (Modified – with permission from [20])
No comments:
Post a Comment