WHOM is going to do the human testing to see if this can be applied to adults and humans? Specific names only, we need to hold people responsible instead of letting everything in stroke slip because there is NO leadership monitoring adherence to completing the 100% recovery stroke strategy. Of course we have NO strategy because there isn't 2 functioning neurons in the stroke medical world.
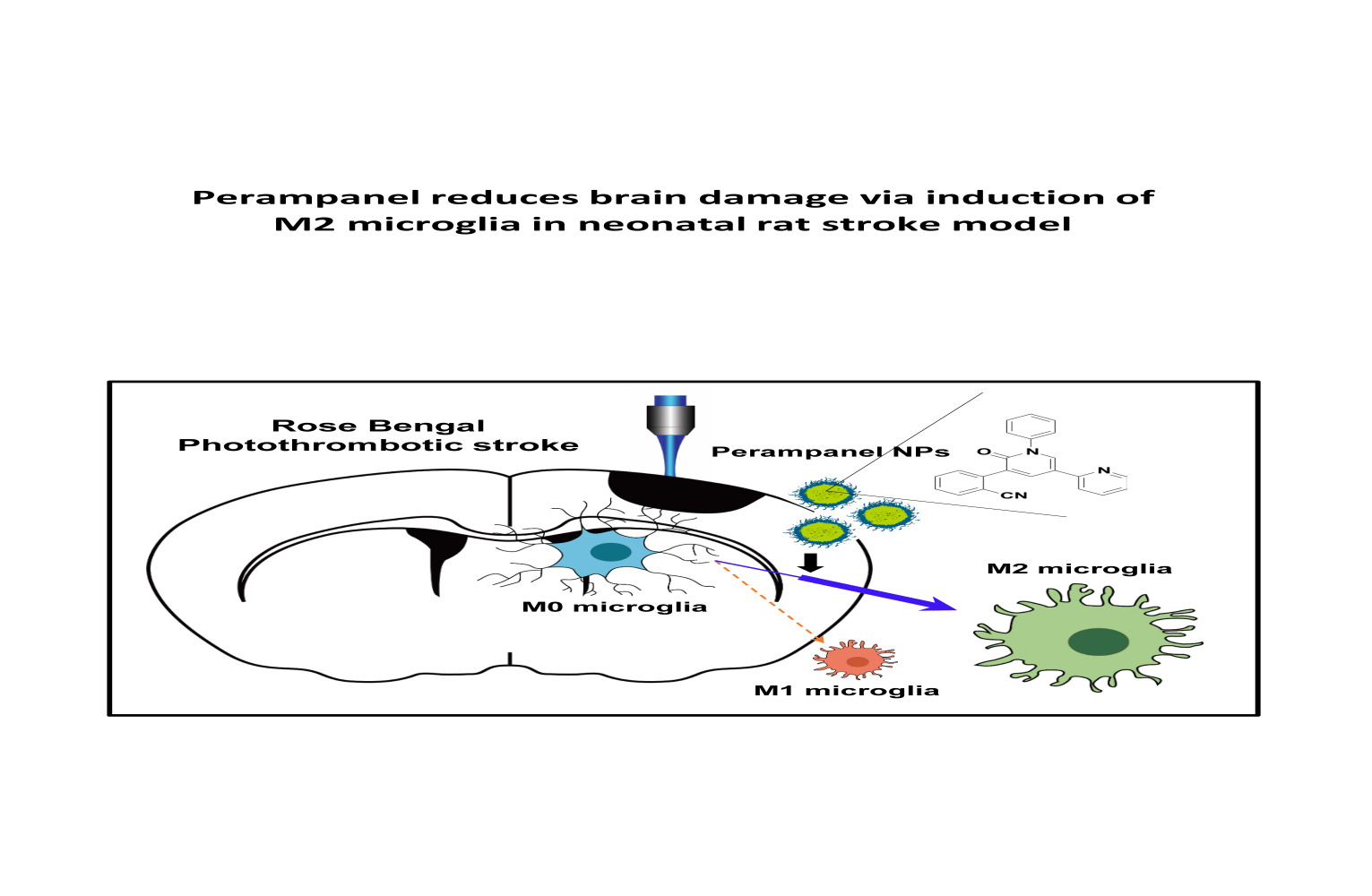
Perampanel Reduces Brain Damage via Induction of M2 Microglia in a Neonatal Rat Stroke Model
Authors Shin HJ, Lee KY, Kang JW, Choi SG, Kim DW , Yi YY
Received 6 February 2022
Accepted for publication 18 June 2022
Published 27 June 2022 Volume 2022:17 Pages 2791—2804
DOI https://doi.org/10.2147/IJN.S361377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Hyo Jung Shin,1,2,* Ka Young Lee,1,3,* Joon Won Kang,4,5 Seung Gyu Choi,1,5 Dong Woon Kim,1,2,4,* Yoon Young Yi6,*
1Department of Anatomy and Cell Biology, Chungnam National University, Daejeon, Republic of Korea; 2Brain Research Institute, Chungnam National University, Daejeon, Republic of Korea; 3Department of Rehabilitation Medicine, Seoul National University Bundang Hospital, Seongnam, Republic of Korea; 4Department of Medical Science, Chungnam National University, Daejeon, Republic of Korea; 5Department
of Pediatrics, Chungnam National Hospital, School of Medicine, Chungnam
National University, Daejeon, Republic of Korea; 6Department of Pediatrics, College of Medicine, Hallym University and Gangdong Sacred Heart Hospital, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Dong Woon Kim; Yoon Young Yi, Tel +82-42-580-8207 ; +82-2-2224-2251, Email visnu528@cnu.ac.kr; 070133@kdh.or.kr
Purpose:
Ischemic stroke is a leading cause of death and disability worldwide.
Additionally, neonatal ischemia is a common cause of neonatal brain
injury, resulting in cerebral palsy with subsequent learning
disabilities and epilepsy. However, there is currently a lack of
effective treatments available for patients with perinatal ischemic
stroke. In this study, we investigated the effect of perampanel
(PER)-loaded poly lactic-co-glycolic acid (PLGA) by targeting microglia
in perinatal stroke.
Methods: After formation of
focal ischemic stroke by photothrombosis in P7 rats, PER-loaded PLGA was
injected intrathecally. Proinflammatory markers (TNF-α, IL-1β, IL-6,
COX2, and iNOS) and M2 polarization markers (Ym1 and Arg1) were
evaluated. We investigated whether PER increased M2 microglial
polarization in vitro.
Results: PER-loaded PLGA
nanoparticles decreased the pro-inflammatory cytokines compared to the
control group. Furthermore, they increased M2 polarization.
Conclusion:
PER-loaded PLGA nanoparticles decreased the size of the infarct and
increased motor function in a perinatal ischemic stroke rat model.
Pro-inflammatory cytokines were also reduced compared to the control
group. Finally, this development of a drug delivery system targeting
microglia confirms the potential to develop new therapeutic agents for
perinatal ischemic stroke.
Graphical Abstract:
Keywords: ischemic stroke, neonate, poly lactic-co-glycolic acid, PLGA, nanoparticle, perampanel, microglial polarization
Graphical Abstract:
Introduction
Perinatal ischemic stroke occurs in 1 per 4000 live births1 and causes significant morbidity and severe long-term neurologic sequelae in children. The pathophysiology of perinatal stroke may often relate to the unique environmental factors that surround the time of birth, with a relatively low recurrence rate, in contrast to adult cases.2 For the treatment of ischemic stroke, recanalization of blood vessels with thrombolytic or endovascular treatments can be helpful.3,4 However, this has a narrow therapeutic window of 4.5 hours from the onset of a stroke in certain eligible patients, and it is currently rarely performed in newborns.5 Thus, there is still no standard acute therapy available for perinatal stroke, and the focus is on supportive treatment and neuroprotection. It is important to investigate effective treatments for perinatal ischemic stroke.
In response to ischemic stroke, microglia are activated and polarized to the pro-inflammatory M1 phenotype or the anti-inflammatory M2 phenotype. Also, pro-inflammatory M1 microglia, as the primary source of cytokines and free radicals, can induce secondary brain damage.6 Control of microglial polarization can be an important therapeutic strategy. The immunomodulatory molecules generated by these microglia, such as cytokines and chemokines, are closely associated with secondary brain damage or repair, respectively, following ischemic stroke. In the MCAO-induced ischemic stroke model, M1 phenotype microglia were increased, and ischemic lesions induced microglial inflammation in the ipsilateral cortex.7 Therefore, investigating microglial changes and their functions is crucial to understanding ischemic stroke pathophysiology.
Nanoparticles have been widely applied in biomedical fields for use in diagnosis, biosensing, and drug delivery. Recently, many nanoparticles have been reported to be useful vehicles for delivery of drugs to inhibit the over-activation of microglia and neuro-inflammation.8,9 Biodegradable polymeric nanoparticles such as poly lactic-co-glycolic acid (PLGA) and poly lactic acid (PLA) have been well studied as stable drug carriers for drug delivery. Among them, PLGA has proven to be effective as a drug delivery method for gene regulators such as siRNAs, plasmids, and miRNA.10–12 PLGA nanoparticles (NPs) have also been approved for pharmaceutical application by the US Food and Drug Administration.13 Moreover, recent studies have shown that PLGA NPs are preferentially localized to microglia.14 Among the cells in CNS, microglia were shown to take up 63.41% of rhodamine-conjugated PLGA NPs at 24 hours.8,15
Perampanel (PER) is a noncompetitive α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist that is clinically used for seizure control. AMPA receptors are postsynaptic ionotropic excitatory receptors with binding sites for glutamate that are thought to participate in the induction of seizures by synchronizing excitatory glutamatergic signaling.16 In preclinical studies, PER was found to be effective in preventing seizures in a seizure model,17 and was studied in vitro as an inhibitor of the dose-dependent AMPA-mediated increase in intracellular calcium.18 Moreover, recent studies have reported that PER has neuroprotective effects in experimental models of stroke19,20 and traumatic brain injury.21 The AMPA receptor, the target of PER, is present not only in neurons but also in activated microglia.22 By examining the action on the AMPA receptor of PER contained in nanoparticles with high affinity for microglia, it may be possible to target this receptor for perinatal stroke treatment. There is still no research of the action of PER on microglia for the treatment of perinatal stroke. Based on the distribution of drug-loaded PLGA NPs, it is reasonable to hypothesize that PER-loaded NPs will influence microglia directly. In the present study, we investigated the effects of PER-loaded NPs on microglial polarization in a perinatal ischemic stroke rat model.
More at link.
No comments:
Post a Comment