http://journal.frontiersin.org/article/10.3389/fneur.2015.00083/full?
- 1Summit Neurovascular Specialists, Akron, OH, USA
- 2Department of Research, Akron General Medical Center, Akron, OH, USA
- 3Department of Cell Biology and Physiology, University of North Carolina School of Medicine, Chapel Hill, NC, USA
Introduction
The current strategy for acute ischemic stroke (AIS)
treatment is based on two pillars: time from ictus to revascularization
(TIR) and revascularization success as measured by the modified
thrombolysis in cerebral ischemia scale (mTICI). The assumption is that
clinical outcome following AIS is dependent on the interaction of these
two factors. The shorter the TIR and the higher the mTICI, the better
the outcome. It follows that the strategy behind current intra-arterial
treatment for acute ischemic stroke (IAT-AIS) is the faster and more
complete the revascularization, the better the clinical outcome.
However, despite the recent impressive improvement in revascularization
rates and decrease in time to revascularization, until recently the
clinical improvement rate remained unchanged at approximately 40–45%
(Table 1) with a ratio of good clinical outcome (GCO) in treatment vs. control arms of approximately 1.7 (1–11).
Recent trials have published GCOs above 50% in the treatment arm, but
with the same ratio of GCOs between the treated and untreated arms
around 1.7 (12, 13). How we can explain this consistency? A fresh look at our strategy and selection criteria is obviously warranted.
TABLE 1
Physiological Background and the 50% Barrier
Normal cerebral blood flow (CBF) is 50–55 ml/100 g/min (14, 15). AIS induces a rapid and sustained reduction in CBF. Clinical signs of ischemia generally become apparent when CBF drops below 23 ml/100 g/min (16). If residual CBF (rCBF) further decreases to 15–16 ml/100 g/min, the cortical-evoked potential ceases within seconds (16). The rate of depression of the evoked potential (EP) amplitude (expressed in units of percent of control/min) is highly correlated with the residual flow, following a linear relationship with the regression line intercepting the flow axis at 15.2 ml/100 g/min (17). The data strongly suggest a threshold-like relationship also exists between the amplitude of the EP and local blood flow. If flow is greater than approximately 16 ml/100 g/min the EP is not affected, but at flows less than approximately 12 ml/100 g/min the EP is abolished (17). Neither the clinical signs of ischemia nor cessation of the EP is synonymous with cell death, but cessation of the EP is one of the final stages before irreversible injury (infarction). Its physiological purpose is to conserve energy by decreasing cell metabolism to the minimal level possible; however, cell death ensues thereafter.
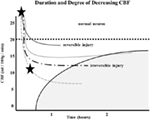
Similarly, the relationship between time to irreversible damage and rCBF is well-documented (18). In one study, rCBF in monkeys was measured in the ischemic area with time after occlusion until irreversible tissue damage occurred (16). An infarction threshold was observed relating the rCBF to time between the initial drop in CBF to irreversible ischemia (Figure 1). This work confirmed prior studies using the neuronal EP and showed that when rCBF reached a low level of around 10 ml/100 g/min, the available time to salvage the brain tissue was extremely short (<1 h) (16).
FIGURE 1
Figure 1. Depth of ischemia and time to irreversible cerebral damage: time to irreversible cerebral damage depends on the depth of ischemia, which depends on the pial collateral supply to the ischemic territory. Since different patients have different collaterals, the depth of ischemia will vary among patients, as will the time available for therapy to salvage the tissue (16).
The depth of ischemia, i.e., the level of rCBF, will vary from patient to patient depending on the available retrograde pial collaterals to the ischemic area. The major determinants of the amount of collateral perfusion are the number and diameter of these pial collaterals, plus perfusion pressure and resistance above and below the collateral network. Greater collateral numbers and diameters sustain a higher rCBF, thus more salvageable brain and a smaller final infarct volume.
Following AIS, rCBF stays virtually unchanged if spontaneous recanalization of the occluded blood vessels does not occur (16, 18, 19). While the clinical symptoms of ischemia will often resolve if CBF is restored promptly, prolonged low levels of rCBF leads to irreversible brain tissue damage. Since the time of ischemia that the brain tissue can tolerate before irreversible damage ensues depends on the rCBF value, which is patient-specific and highly dependent on the collaterals, it follows that every patient has his or her own time (Figure 1) (16, 18, 19). Hence, if we correctly select patients that are optimal candidates (patients with ischemic but viable tissue) and are able to achieve safe, full, and timely revascularization (prior to irreversible ischemic damage occurring), the clinical symptoms of a stroke should improve significantly and rather quickly.
Given this information, the most logical explanation for the remarkably consistent results of the different IAT-AIS trials, with <50% GCOs (modified Rankin Score, or mRS, ≤2), is that around half of treated patients have poor pial collaterals, thus causing them to have a relatively low rCBF such that they enter into irreversible ischemia before therapy can be administered, even when timely (within 6 h) revascularization is achieved. This observation implies a potential ceiling effect for IAT-AIS; we call this phenomenon the 50% barrier (Figure 2).
More at link.
 Firas Al-Ali
Firas Al-Ali John J. Elias
John J. Elias
No comments:
Post a Comment