Our fucking failures of stroke associations should jump at the chance to use this in stroke and create protocols for its use. But nothing will occur because they don't give a shit about survivors. Look at their sites, NOTHING USEFUL FOR SURVIVORS. Just crappy guidelines, press releases about prevention, and F.A.S.T.
Traumatic brain injury neuroelectrochemical monitoring: behind-the-ear micro-instrument and cloud application
Journal of NeuroEngineering and Rehabilitation volume 17, Article number: 114 (2020)
Abstract
Background
Traumatic Brain Injury (TBI) is a leading cause of fatality and disability worldwide, partly due to the occurrence of secondary injury and late interventions. Correct diagnosis and timely monitoring ensure effective medical intervention aimed at improving clinical outcome. However, due to the limitations in size and cost of current ambulatory bioinstruments, they cannot be used to monitor patients who may still be at risk of secondary injury outside the ICU.
Methods
We propose a complete system consisting of a wearable wireless bioinstrument and a cloud-based application for real-time TBI monitoring. The bioinstrument can simultaneously record up to ten channels including both ECoG biopotential and neurochemicals (e.g. potassium, glucose and lactate), and supports various electrochemical methods including potentiometry, amperometry and cyclic voltammetry. All channels support variable gain programming to automatically tune the input dynamic range and address biosensors’ falling sensitivity. The instrument is flexible and can be folded to occupy a small space behind the ear. A Bluetooth Low-Energy (BLE) receiver is used to wirelessly connect the instrument to a cloud application where the recorded data is stored, processed and visualised in real-time. Bench testing has been used to validate device performance.
Results
The instrument successfully monitored spreading depolarisations (SDs) - reproduced using a signal generator - with an SNR of 29.07 dB and NF of 0.26 dB. The potentiostat generates a wide voltage range from -1.65V to +1.65V with a resolution of 0.8mV and the sensitivity of the amperometric AFE was verified by recording 5 pA currents. Different potassium, glucose and lactate concentrations prepared in lab were accurately measured and their respective working curves were constructed. Finally,the instrument achieved a maximum sampling rate of 1.25 ksps/channel with a throughput of 105 kbps. All measurements were successfully received at the cloud.
Conclusion
The proposed instrument uniquely positions itself by presenting an aggressive optimisation of size and cost while maintaining high measurement accuracy. The system can effectively extend neuroelectrochemical monitoring to all TBI patients including those who are mobile and those who are outside the ICU. Finally, data recorded in the cloud application could be used to help diagnosis and guide rehabilitation.
Background
Traumatic brain injury (TBI) is a non-degenerative, non-congenital condition; it could be defined as a set of perceptible and non-perceptible brain insults due to an external impact on the head. Such insults include brain herniation, haemorrhage and contusion [1, 2]. In addition to the primary injury that occurs at the moment of impact, secondary injuries are likely to develop especially in mild and severe TBI injuries [3]. These secondary injuries may take hours or even days to manifest and can lead to reduction in life expectancy, altered level of consciousness, post-traumatic disorder and neurological disorders, along with other cognitive and psychological/psychiatric impairments [3]. Hence, TBI should not be viewed as a single event but rather as a sustained condition calling for monitoring, supervision and rehabilitation [4].
TBI is characterised by a complex pathway where patients could relapse after surgery, while in the intensive care unit (ICU), the high dependency unit (HDU) or in normal hospital wards, and may require additional invasive interventions. The motivation for neuroelectrochemical monitoring of TBI patients stems from the need for timely neurological intervention and prevention of adverse effects. However, currently, TBI neuroelectrochemical monitoring is limited to fully sedated patients in the ICU who have undergone craniotomy neurosurgery as a result of sustaining a severe TBI and/or showing acute brain insults [5, 6].
A closely monitored event in TBI is the onset of spreading depolarisations (SDs), also referred to as “brain tsunamis”. These are slow changing mass depolarization waves that originate from the lesion foci and spread out to neighbouring tissues at risk of secondary injury [7]. SDs are strongly associated with poor outcome in TBI patients and are measured by means of electrocorticography (ECoG). Subdural strip electrodes are traditionally used to monitor SD events, however, they require the patient to undergo craniotomy. Recently, intraparenchymal electrodes were used to accurately monitor these events; these electrodes are inserted via burr hole which is a minimally-invasive procedure [8].
Chemical monitoring of the injured brain can give an indication of tissue health and metabolism [9]. In 2014, a consensus statement from the International Microdialysis Collaborative Group identified glucose and the lactate/pyruvate (L/P) ratio as the most relevant neurochemical biomarkers in TBI monitoring [10]. Glucose concentration reflects local metabolism, hence, poor outcomes have a direct link to low glucose concentrations (< 0.8 mM at 0.3 l/min in tissue interstitial space) [11–15]. Likewise, the association of abnormally high glucose concentrations with poor outcome has also been reported [16, 17] reflecting failure of glucose metabolism due to local tissue death. Absolute concentrations of lactate and pyruvate, in conjunction with the L/P ratio provide information about the cellular redox state in the area of interest. Relatively high concentrations of lactate can be due to both ischemic and non-inshemic (e.g. mitochondrial dysfunction) causes [18, 19]. Continuous on-line microdialysis (co-MD) is used for sampling brain extracellular fluid to measure neurochemical biomarker concentrations and changes [6]. A single microdialysis probe perfused with a physiological solution is inserted into the monitored region either during craniotomy or through cranial bolt in such a way as to cause minimum tissue disruption. Intraparenchymal ECoG electrodes can be inserted through the same bolt as the microdialysis probe which leads to minimally-invasive monitoring [8]. Potassium measurement should also be included in order to enable a spatiotemporal correlation between the chemical and electrical measurements: potassium measurements can chemically denote the onset of SDs [20].
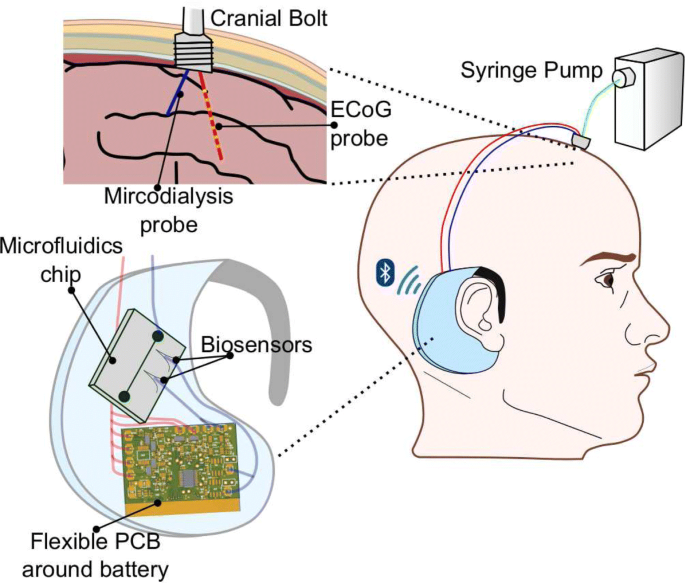
In summary, the device has to measure the biopotential ECoG signals with a resolution sufficient to identify the SDs and make inference on the depolarization of the injured brain [6]. It also has to support amperometry to measure glucose and lactate, as well as potentiometry to measure potassium. This article presents a complete system for monitoring TBI that consists of a wearable bioinstrument (Fig. 1) and a cloud application for data visualisation and analysis.
The setup of the proposed behind-the-ear wearable device. The solution consists of: the micro-instrument (flexible PCB), microfluidic chip and biosensors. The device connects to a minimally-invasive cranial bolt, which is fixed on the patient’s head where the injury is. The bolt has two lumens, one for an ECoG probe and the other for the microdialysis probe. The latter requires a syringe pump to perfuse the probe membrane. The device is wireless and supports bluetooth low-energy (BLE) protocol
The lack of an affordable, wearable, relatively non-invasive instrument is the major barrier for real-time neuroelectrochemical monitoring of patients that are mobile and in risk of secondary injuries; or patients in low-income countries, military and “curb-medicine” settings. Several studies have reported instruments for brain monitoring. An integrated chip for wireless neurochemical measurement was proposed by Roham et al. (2008) [21]. The chip offered both amperometry and fast-scan cyclic voltammetry, However, it had a limited number of channels and a reduced resolution at high sampling rate. In the same vein, Kasasbeh et al. (2013) presented a device that is also limited to neurochemical measurements. Additionally, it is not wearable and is instead designed to be attached to neurosurgical stereotactic frames [22]. In contrast, Piangerelli et al. (2014) proposed an invasive instrument restricted to cortical/electrical signals [23]. Other studies in the literature focused on the design of probes aimed at improving the usability and wearablity of TBI instruments [24].
Pivoting to instruments particularly designed for TBI or neuroelectrochemical monitoring, Papadimitriou et al. (2016) developed a high-performance two board solution. The boards, one for biopotential recording and the other for chemical biomarker measurement, were designed to realise an ambulatory bedside equipment, thus it employed a wired connection to a data collection system and occupied an area of around 400 cm2 per board [5]. In Zafeiropoulos, Papadimitriou et al. (2018), PANACEA, an integrated instrument for cortical and neurochemical signals was developed. Most notably, the instrument could be connected to a receiver either through a wired connection or wirelessly via IEEE 802.15.4 (Zigbee) protocol. The latter employing an external antenna had a sampling rate up to 1 ksps/channel and traded power for performance [25]. Again, the instrument, though portable, was not designed as a wearable solution and remained somewhat too large (around 80 cm2) to be wearable.
In comparison to ambulatory bed-side instruments, a wearable instrument would allow shorter connection tubes between the patient and the analysis system. This would also facilitate real-time monitoring and enhance temporal resolution because sampled chemical biomarkers would reach the instrument faster. Pagkalos’s “LENBIC” (Low-power Electrical and Neurochemical Biosensor Interfacing Chip) is a high-performance application specific integrated circuit for TBI [26]. LENBIC offers dramatic footprint reduction (7.5 mm2), high accuracy and ultra-low power performance. However, the chip - being an analog front-end (AFE) - requires additional peripherals, such as embedded controller and integrated wireless transceivers, which increase the device footprint and cost.
What we present here is a wearable micro-instrument specific for full neuroelectrochemical TBI monitoring that trades off performance for an optimal balance between size and cost. This is done while keeping the performance high enough to accurately and sufficiently monitor TBI related biomarkers and events. This trade-off is depicted in Fig. 2 with respect to the other instruments described above. In this article, the performance of the prototype has been validated using bench testing.
No comments:
Post a Comment