You'll have to ask your doctor why the hell edaravone is approved in Japan since 2001 but not the US.
Has your stroke hospital done anything with edaravone in the last decade?
edaravone (12 posts to November 2011)
Ebselen, an anti-inflammatory antioxidant, was originally developed by Daiichi Sankyo, in Japan, to treat patients who had suffered a stroke. But the compound was never marketed and has since come off patent. It’s also part of the National Institutes of Health Clinical Collection—several hundred small molecules that have, to some extent, gone through the gamut of human clinical trials and have been found to be safe, but never reached final FDA approval.
ebselen (10 posts to December 2012)
Carnosic Acid Shows Higher Neuroprotective Efficiency than Edaravone or Ebselen in In Vitro Models of Neuronal Cell Damage
1
Maj Institute of Pharmacology, Polish Academy of Sciences, Department of Experimental Neuroendocrinology, 31-343 Krakow, Poland
2
Jerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences, 30-239 Krakow, Poland
*
Author to whom correspondence should be addressed.
Molecules 2024, 29(1), 119; https://doi.org/10.3390/molecules29010119
Original submission received: 19 October 2023
/
Resubmission received: 16 November 2023
/
Revised: 21 December 2023
/
Accepted: 22 December 2023
/
Published: 24 December 2023
Abstract
This study compared the neuroprotective efficacy of
three antioxidants—the plant-derived carnosic acid (CA), and two
synthetic free radical scavengers: edaravone (ED) and ebselen (EB)—in in
vitro models of neuronal cell damage. Results showed that CA protected
mouse primary neuronal cell cultures against hydrogen peroxide-induced
damage more efficiently than ED or EB. The neuroprotective effects of CA
were associated with attenuation of reactive oxygen species level and
increased mitochondrial membrane potential but not with a reduction in
caspase-3 activity. None of the tested substances was protective against
glutamate or oxygen-glucose deprivation-evoked neuronal cell damage,
and EB even increased the detrimental effects of these insults. Further
experiments using the human neuroblastoma SH-SY5Y cells showed that CA
but not ED or EB attenuated the cell damage induced by hydrogen peroxide
and that the composition of culture medium is the critical factor in
evaluating neuroprotective effects in this model. Our data indicate that
the neuroprotective potential of CA, ED, and EB may be revealed in
vitro only under specific conditions, with their rather narrow
micromolar concentrations, relevant cellular model, type of toxic agent,
and exposure time. Nevertheless, of the three compounds tested, CA
displayed the most consistent neuroprotective effects.
1. Introduction
Oxidative
stress has long been recognized as the pivotal component of neuronal
death in both acute (stroke, traumatic brain injury) and chronic
neurodegenerative dis-eases, e.g., Alzheimer’s, Parkinson’s and
Huntington’s disease [1,2,3].
It has been well established that oxidative stress results from a
disturbed balance between the excessive intracellular accumulation of
reactive oxygen species (ROS) and reactive nitrogen species (RNS) and
endogenous antioxidant defense system in which glutathione peroxidase,
glutathione reductase, superoxide dismutase, and catalase play the
critical role [4].
The ROS and RNS in high concentrations are directly damaging factors
for lipids, carbohydrates, amino acids, proteins and nucleic acids, in
this way disrupting intracellular organelles, structural proteins and
membranes [5,6].
Therefore, the removal of pathologically produced free radicals has
been proposed as a viable neuroprotective strategy. Besides
anti-oxidative enzymes, vitamins A, C and E, glutathione, plant
polyphenolic compounds including flavonoids, thioredoxin,
metallothionein, ceruloplasmin, and some trace elements can alleviate
the harmful effects of ROS and RNS [2].
Although natural antioxidants show high activity in the scavenging of
free radicals, their bioavailability is limited by low absorption and
poor stability [7].
Regarding synthetic antioxidants, some compounds with strong free
radical scavenging properties or free radical trapping activities (e.g.,
NXY-059—disufenton sodium and its derivatives) showed only modest
neuroprotective activity and a bell-shaped dose–response curve in in
vivo experimental models of neuronal damage. Moreover, in clinical
trials, they failed to show consistent neuroprotective effects over
placebo [8].
It should be mentioned here that clinical trials on the neuroprotective
potential of antioxidants were conducted among small study populations [3].
On the other hand, some antioxidative compounds such as gallic acid
esters, hydroxytoluene, and butylated hydroxyanisole display undesired
effects on living organisms [9].
Among antioxidants with potential translational value, low molecular
weight, and cell membrane-permeable superoxide dismutase mimetics, such
as the nitroxide tempol
(4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl), seem quite promising [10].
The inconsistent results of studies on the neuroprotective effects of
antioxidants are thought to be due to unfavorable pharmacokinetic
profiles, i.e., low water solubility and bioavailability, difficult
penetration through the blood–brain barrier (BBB), uncertain stability,
and insufficient knowledge of their metabolism and elimination. Another
problem concerns establishing therapeutic concentrations of antioxidants
in blood and brain tissue because, depending on their concentrations,
these compounds may exert antioxidative or prooxidative effects. One of
the methods to improve the pharmacokinetic and pharmacodynamic
properties of antioxidants is their encapsulation in nanoparticles
(nanocarriers) [11,12].
However, before this step, it is essential to select the most promising
antioxidant among various candidates in the same screening platforms
for neuroprotection.
Based on the literature
search, we have chosen three hydrophobic compounds with antioxidant
properties: edaravone, ebselen, and carnosic acid. Edaravone (ED,
MCI-186, 3-methyl-1-phenyl-2-pyrazolin-5-one, Figure 1A)
is a clinical drug developed by Mitsubishi Tanaba (Osaka, Japan) and
has been approved by Japan and the FDA for ALS treatment since 2015 and
2017, respectively [13].
It is a free radical scavenger with the capacity to mitigate oxidative
injury in various models of neuronal damage. The protective effects of
ED in attenuating NO, glutamate, and hypoxia-induced cytotoxicity and
apoptosis have been reported [14,15,16,17]. ED also effectively protects astrocytes from oxidative stress or infectious insults such as bacterial lipopolysaccharides [18]. Ebselen (EB, 2-phenyl-1,2-benzisoselenazol-3(2H)-one, Figure 1B) is an organoselenium compound with well-characterized toxicology and pharmacology [19].
Its antioxidative mechanism of action involves glutathione
peroxidase-like activity and ability to react with thiols,
peroxynitrites, and hydroperoxides. EB protects cell components from
oxidative damage [20,21].
EB and its analogues showed neuroprotective effects in various
experimental models against cell damage induced by oxygen and glucose
deprivation (OGD), amyloid β(1-42), lipopolysaccharide,
6-hydroxydopamine (6-OHDA), and in MPTP-treated mice [22,23,24,25,26].
Carnosic acid (CA,
4aR,10aS)-5,6-dihydroxy-7-isopropyl-1,1-dimethyl-1,3,4,9,10,10a-hexahydro-2H-phenanthrene-4a-carboxylic
acid, Figure 1C)
isolated from rosemary (Rosmarinus officinalis) and common sage (Salvia
officinalis) possesses antioxidative, anti-inflammatory, and
anti-neoplastic properties [27,28,29].
CA was found to ameliorate oxidative stress-, glutamate-, and
hypoxia-induced injury of neuronal as well as displayed neuroprotective
activity in in vitro and in vivo models of Parkinson’s or Alzheimer’s
disease [30,31,32,33,34,35,36,37,38,39].
Although most of the above-cited studies
unanimously indicate the neuroprotective effects of ED, EB, and CA, they
differ in experimental settings, doses of compounds, times of
exposures, and measurements of cellular damages, etc., which makes their
comparison difficult. Therefore, in order to select the most promising
neuroprotective compound of those three for nanoencapsulation for future
experimental studies, it was necessary to estimate their properties
under similar, well-controlled conditions. Thus, in the present study,
we compared biocompatibility and neuroprotective potentials of ED, EB,
and CA in a wide range of concentrations in mouse primary neuronal cell
cultures exposed to oxidative stress inducer (hydrogen peroxide, H2O2),
excitotoxic factor (glutamate), and OGD. Moreover, some protective
mechanisms were studied for the best-acting neuroprotectant. Finally,
biosafety and neuroprotective profiles of these three compounds were
also tested in the human neuronal-like model: undifferentiated (UN-) and
retinoic acid-differentiated (RA-) neuroblastoma SH-SY5Y cells exposed
to H2O2.
2. Results and Discussion
2.1. The Effect of Edaravone in Primary Neuronal Cell Cultures
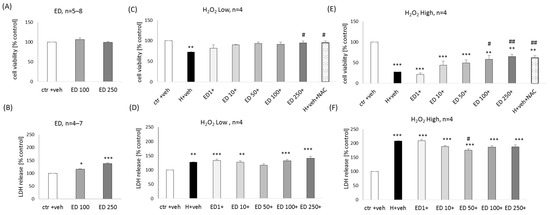
ED at concentrations of 100 and 250 μM did not evoke any reduction in cell viability in primary neuronal cell cultures (Figure 2A) but slightly increased the LDH release (17–37%) (Figure 2B).
A significant neuroprotective effect of ED (100 and 250 μM) was found
in the model of neuronal cell damage induced by lower (150 μM) and
higher (200 μM) concentrations of H2O2 at the
level of the cell viability assessment. This effect was comparable to
protection mediated by positive control, NAC (1 mM) (99.28% and
94.15–105.29% of NAC efficiency for low and high H2O2, respectively) (Figure 2C,E). In the cytotoxicity assay, a slight reduction was observed of the high H2O2-evoked changes in this parameter by ED at a concentration of 50 μM (Figure 2F), but no impact of ED was found on low H2O2-induced LDH release (Figure 2D).
Figure 2.
Biosafety (A,B) and neuroprotection (C–F) assessment against the hydrogen peroxide (H2O2)-induced
cell damage by edaravone (ED) in primary neuronal cell cultures. The
eight days in vitro cortical neurons were treated either with vehicle or
with ED alone (100 and 250 μM) or ED (1–250 μM) in combination with low
(150 μM) or high (200 μM) concentrations of H2O2 for 24 h. An antioxidant N-acetyl-cysteine (NAC, 1 mM) was used as a positive control of the model. Cell viability (A,C,E) and cytotoxicity (B,D,F)
were measured by MTT reduction and LDH release assays, respectively.
The data were normalized to vehicle-treated cells and presented as the
mean ± SEM. The number of independent experiments (n) is indicated in each graph. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. vehicle-treated cells; # p < 0.05 and ## p < 0.01 vs. H2O2-treated cells.
More at link.




No comments:
Post a Comment