You'll have to ask your competent? doctor if anything in here will help with the dendritic spine branching you need to reconnect neurons through the white matter.
If your doctor doesn't have anything on how you can get dendritic branching going; you don't have a functioning stroke doctor! Here's help for your doctor.
dendritic branching (39 posts to February 2012)
dendritic spine formation (6 posts to February 2017)
dendritic spines (19 posts to January 2013)
Cdk5-dependent rapid formation and stabilization of dendritic spines by corticotropin-releasing factor
Translational Psychiatry volume 14, Article number: 29 (2024)
Abstract
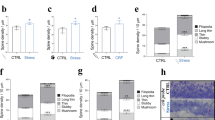
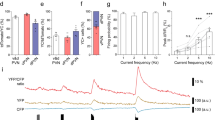
The neuropeptide corticotropin-releasing factor (CRF) exerts a pivotal role in modulating neuronal activity in the mammalian brain. The effects of CRF exhibit notable variations, depending on factors such as duration of exposure, concentration, and anatomical location. In the CA1 region of the hippocampus, the impact of CRF is dichotomous: chronic exposure to CRF impairs synapse formation and dendritic integrity, whereas brief exposure enhances synapse formation and plasticity. In the current study, we demonstrate long-term effects of acute CRF on the density and stability of mature mushroom spines ex vivo. We establish that both CRF receptors are present in this hippocampal region, and we pinpoint their precise subcellular localization within synapses by electron microscopy. Furthermore, both in vivo and ex vivo data collectively demonstrate that a transient surge of CRF in the CA1 activates the cyclin-dependent kinase 5 (Cdk5)-pathway. This activation leads to a notable augmentation in CRF-dependent spine formation. Overall, these data suggest that upon acute release of CRF in the CA1-SR synapse, both CRF-Rs can be activated and promote synaptic plasticity via activating different downstream signaling pathways, such as the Cdk5-pathway.
Similar content being viewed by others
Introduction
Synapse formation and neuronal networks are built and/or strengthened by exogenous factors such as learning and stress [1]. Neuromodulators exert diverse effects on the formation and pruning of synaptic connections [2, 3]. Among these agents, corticotropin-releasing factor (CRF), a neuromodulator intricately linked to synaptic plasticity, plays a central role in the hypothalamic–pituitary–adrenal axis (HPA), which is a stress axis [4, 5]. With the onset of stress, CRF is released in the paraventricular nucleus of the hypothalamus. The HPA axis operates as a feedback-regulating system in which CRF release leads to the synthesis of glucocorticoids [5]. These glucocorticoids suppress the secretion of CRF upon reaching the hypothalamus and higher brain regions [5, 6]. Beyond its role in the HPA axis, CRF is endogenously expressed in discrete regions of the brain, where it regulates dendrite development and maturation, synaptogenesis, and the integration of adult-born neurons into neuronal circuits [7,8,9]. In chronic stress, characterized by prolonged exposure or elevated CRF concentrations, there is a decline in spine density and dendritic complexity [10]. However, our recent findings also underscored the significance of CRF in acute stress situations [11]. CRF modulates excitatory transmission within specific neurons via two receptors: CRF-R1 and CRF-R2 [12, 13]. CRF-R1 and CRF-R2 are classified as type B G protein-coupled receptors. Each CRF-R originates from discrete genes and exhibits multiple splice variants [14]. CRF-R1 and CRF-R2 are ubiquitously expressed in both central and peripheral tissues with a 70% amino acid sequence similarity in their transmembrane and intracellular domains [15, 16]. CRF-Rs can be activated by various stimuli, including stress, CRF, and CRF-related peptides such as urocortin 1, urocortin 2 (or stresscopin-related peptide), and urocortin 3 (or stresscopin). Binding of CRF-R agonits with the extracellular domains of CRF-R1 and R2 induces structural alterations in these receptors, ultimately activating G-proteins and initiating downstream signaling cascades [17].
The distribution of CRF and CRF-Rs varies between specific areas of the brain. Due to CRF’s neuromodulatory nature it can induce volumetric remote actions, therefore the localization of CRF-Rs can differ from its release sites [18]. While previous studies have reported the presence of CRF in a subpopulation of GABAergic interneurons in the hippocampus and CRF-Rs in excitatory synapses of the pyramidal cells (PCs), precise subcellular localization remains debated. Previous reports documented the presence of CRF-R2 in the hippocampus but in lower amounts than CRF-R1 [18, 19]. Interestingly, acute CRF exposure demonstrates an increase in calcium-dependent vesicular release and an increase in the active zone [11], which are modifications happening in the presynaptic compartment. In addition, both CRF-Rs influence spine type, and its activation enhances long-term plasticity, as seen in long-term potentiation (LTP) measurements documented in previous observations [11]. However, CRF-R2 expression and its role in the hippocampus remain unclear. Using different labeling techniques and electron microscopy, we demonstrate the exact localization of CRF, CRF-R1, and CRF-R2 in the CA1-SR and identify long-term effects of acute CRF exposure, such as an increase in mature spines with enduring stability over hours. Notably, both CRF-Rs are present in the hippocampus, regulating CRF actions in synapse morphology and function. The molecular mechanisms behind CRF peptide’s regulation of spine density, although not fully elucidated, likely involve changes in the actin cytoskeleton. One of the promising candidates in the CRF-dependent regulation of spine formation is Cdk5, a serine/threonine kinase vital for neuronal development, spine formation, learning, and memory [20, 21, 22].
This study utilizes two-photon ex vivo dendritic spine imaging and in vivo stereotactic injections to reveal the transient and stabilizing effects of acute CRF in the CA1-SR synapses. It further showcases the presence of CRF-R1 and CRF-R2 and localization in CA1 synapses, in driving CRF-induced spine increase. Altogether, this work indicates CRF’s role in spine formation, stabilization, and cytoskeletal rearrangements through the activation of the Cdk5 pathway.

No comments:
Post a Comment