I have zero understanding of this but you will want your doctor to know what to do about this because of your heightened risks of dementia. Assuming that your doctor even knows about those risks.
Your chances of getting dementia.
1. A documented 33% dementia chance post-stroke from an Australian study? May 2012.
2. Then this study came out and seems to have a range from 17-66%. December 2013.`
3. A 20% chance in this research. July 2013.
4. Dementia Risk Doubled in Patients Following Stroke September 2018
But maybe this: which you doctor needs to come up with EXACT amounts of caffeine to consume. YOUR DOCTOR'S RESPONSIBILITY!
Caffeine causes widespread brain entropy (and that's a good thing)
April 2018
The latest here:
Brain Entropy Mapping in Healthy Aging and Alzheimer’s Disease
- Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, United States
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, for which aging remains the major risk factor. Aging is under a consistent pressure of increasing brain entropy (BEN) due to the progressive brain deteriorations. Noticeably, the brain constantly consumes a large amount of energy to maintain its functional integrity, likely creating or maintaining a big “reserve” to counteract the high entropy. Malfunctions of this latent reserve may indicate a critical point of disease progression. The purpose of this study was to characterize BEN in aging and AD and to test an inverse-U-shape BEN model: BEN increases with age and AD pathology in normal aging but decreases in the AD continuum. BEN was measured with resting state fMRI and compared across aging and the AD continuum. Associations of BEN with age, education, clinical symptoms, and pathology were examined by multiple regression. The analysis results highlighted resting BEN in the default mode network, medial temporal lobe, and prefrontal cortex and showed that: (1) BEN increased with age and pathological deposition in normal aging but decreased with age and pathological deposition in the AD continuum; (2) AD showed catastrophic BEN reduction, which was related to more severe cognitive impairment and daily function disability; and (3) BEN decreased with education years in normal aging, but not in the AD continuum. BEN evolution follows an inverse-U trajectory when AD progresses from normal aging to AD dementia. Education is beneficial for suppressing the entropy increase potency in normal aging.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that has impacted millions of elderly people but still remains incurable (Ferri et al., 2005; Reitz and Mayeux, 2014). Although AD has been well characterized by AD pathology and clinical symptoms, a major barrier to research progress is the unclear mechanism for how and when normal aging progresses into AD dementia (Kumar and Singh, 2015; Mehta and Yeo, 2017) and why AD symptoms often emerge many years later than AD pathology. This pathology vs. symptom discrepancy (Jack et al., 2010; Jack and Holtzman, 2013) suggests that there may exist a reserve of brain function according to the seminal “cognitive reserve” (CR; Stern, 2006; Stern et al., 2018) model. This reserve may compensate brain damage–induced functional abnormalities in normal aging but fails to do that after disease conversion. To characterize the brain function reserve, we need a more tangible proxy. One candidate is the resting-state brain activity which matches the latent function reserve in two perspectives: first, it is an ongoing process non-specific to any overt brain function; second, it has been postulated to play a role in brain function facilitation (Raichle et al., 2001; Raichle and Gusnard, 2002; Raichle, 2011). Resting-state fMRI (rsfMRI) represents the most widely used tool for studying resting brain activity and has been used to assess neural correlates of brain reserve through the inter-regional functional connectivity (FC) analysis (Arenaza-Urquijo et al., 2013; Bozzali et al., 2015; Marques et al., 2016; Franzmeier et al., 2017; Li et al., 2020). An overall picture revealed by these studies is that higher CR measures are related to stronger FC in distributed brain regions including the default mode network (DMN) area and weaker FC in other restricted focal regions. Because FC is defined by the inter-regional signal correlation in the seed-based FC (Biswal et al., 1995) or the associations to a common temporal fluctuation pattern in the spatial independent component decomposition (Calhoun et al., 2001; Hyvärinen et al., 2001; Beckmann and Smith, 2004), it does not tell anything specific to regional brain activity.
In this study, we proposed entropy of each local voxel as a regional proxy of brain reserve. Entropy is a physical measure for a dynamic system with high entropy indicating less order and more irregularity. It may be informative for delineating the aforementioned AD pathology vs. symptom discrepancy because aging is known to have progressive brain deteriorations (Hayflick, 2004; Drachman, 2006) which inevitably increase the brain entropy. High entropy corresponds to low temporal coherence, which is detrimental to brain functional organization and has to be counteracted to keep the normal brain functionality. Because brain reserve is defined by brain function facilitation and compensation, assessing entropy of functional brain activity may provide a direct outcome measure of the latent brain reserve. In a pilot study (Wang, 2020a,b; full article under separate review) based on data from 862 healthy adults from the human connectome project (Van Essen et al., 2013), we found that brain entropy (BEN) in the DMN (including precuneus, bilateral parietal cortex, and part of temporal cortex) and the executive control network (ECN; including the dorsolateral prefrontal cortex and lateral parietal cortex) increases with age but decreases with education years (an indicator of cognitive reserve for compensating brain dysfunctions) and that lower BEN in DMN and ECN is associated with better performance of cognitive functions. These data suggest the feasibility of BEN for characterizing the latent brain reserve compensation outcome. Although the compensation may be sufficient in normal aging, they may become insufficient when disease progresses, which can reciprocally trigger reserve overactions, leading to a catastrophic reduction of BEN as found in previous biophysiological recording–based AD entropy studies (Stam et al., 2003; Jeong, 2004; Abásolo et al., 2006; Gómez and Hornero, 2010; Mizuno et al., 2010; Yang et al., 2013). To explain this apparent opposed entropy change pattern in normal aging and AD, we proposed a heuristic BEN model as shown in Figure 1. This model considers low BEN in DMN and ECN as beneficial for normal aging because low brain entropy corresponds to high temporal coherence which is evidenced to be important for brain function (Buzsáki and Draguhn, 2004; Buzsaki, 2006; Schroeder and Lakatos, 2009; Saleh et al., 2010; Buzsáki and Watson, 2012; Henry and Obleser, 2012; Lega et al., 2012; Thut et al., 2012; Calderone et al., 2014; Reinhart and Nguyen, 2019). However, in AD, our model predicts a detrimental large BEN reduction in DMN/ECN, indicating a failure of the functional compensation role of brain reserve in AD (Stern, 2006, 2012; Stern et al., 2018). The accumulating brain errors or deteriorations will increase BEN and the risk of brain dysfunction if no compensations occur. This potency, however, can be substantially counteracted by brain reserve or other compensatory mechanisms, resulting in a slowly increasing and then flat topping BEN evolution curve in normal aging (the dashed blue line in Figure 1). When the BEN increase latency reaches a critical point where brain dysfunction cannot be fully compensated anymore, reserve overaction may be triggered, leading to an apparent BEN reduction (the red solid curve in Figure 1). When disease progresses, BEN reduction may be accelerated further by other detrimental factors such as the accumulation of Aβ deposition and perfusion deficits. Both Aβ decomposition and hypoperfusion may cause or be associated with BEN reductions through the CBF vs. brain coherence associations: lower CBF correlates with higher brain activity coherence (Sharbrough et al., 1973; Foreman and Claassen, 2012; higher coherence corresponds to lower BEN).
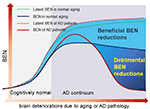
Figure 1. A hypothetical brain entropy (BEN) model for normal aging and Alzheimer’s disease (AD). The dotted line shows the latent BEN evolution trend as a result of the aging-related accumulated brain deteriorations. The dashed line represents the actual BEN evolution curve after brain reserve compensation which imposes negative entropy brings down the total BEN. Catastrophic BEN reduction may start at the disease conversion time due to a potential overaction of the brain reserve.
The main purpose of this study was to assess the feasibility of BEN as an outcome measure of the latent brain function reserve and to evaluate the hypothetical BEN model by leveraging the relatively large data from the AD Neuroimaging Initiative (ADNI)1 and our recently developed rsfMRI-based BEN mapping tool (Wang et al., 2014). The model was assessed using the cross-sectional ADNI rsfMRI data. We hypothesized that AD patients have lower BEN than cognitively healthy elderlies; BEN increases with age in normal aging but not in AD. The association of BEN to function reserve was examined through the correlation between BEN and education, cognitive function measures, and AD pathology measures. Education is a main contributing factor of cognitive reserve (Stern et al., 2018). Longer education years have been demonstrated to be beneficial for combating cognitive impairments. In accordance with the BEN model, we hypothesized that longer education years are associated with reduced BEN in normal aging but not in AD. The entire study reported in this paper is a full expansion of a small sample-based preliminary study (Li and Wang, 2016).
 Ze Wang
Ze Wang
No comments:
Post a Comment