http://journal.frontiersin.org/article/10.3389/fnhum.2015.00246/full?
- 1Brain Rehabilitation Research Center, Malcom Randall VA Medical Center, North Florida/South Georgia Veterans Health System, Gainesville, FL, USA
- 2Department of Aging and Geriatric Research, University of Florida, Gainesville, FL, USA
Introduction
Safe and independent mobility function at home and in the
community requires well-coordinated control of walking. A hallmark of
this healthy control of walking is automaticity, which is the ability of
the nervous system to successfully coordinate movement with minimal use
of attention-demanding executive control resources. The term
“automaticity” is fairly common in literature about control of walking
(for example Paul et al., 2005; Hallett, 2008; Bridenbaugh and Kressig, 2011; Fasano et al., 2012).
However, it often defined loosely and presented in a theoretical
context rather than as a tangible property of locomotor control that can
be evaluated and intervened upon. This is a potential oversight that
may be detrimental to achieving optimal recovery of mobility function in
a variety of clinical populations. Accordingly, this review article
seeks to consolidate evidence from multiple domains of neuroscience and
rehabilitation in order to advance the science of automaticity of
walking. This will fill a gap in the literature by providing a unifying
discussion of automaticity that spans the topics of functional
significance, neurophysiological determinants, measurement, mechanisms
of impairment, and strategies for rehabilitation.
Functional Significance of Automaticity vs. Executive Locomotor Control
Control of walking is seldom, if ever, purely under the
control of either automatic or executive control processes. Rather,
there is a balance between the two processes that is dependent upon the
demands of the task and the capabilities of the individual. This balance
has extremely important implications for the efficacy and safety of
task performance. Research by Shiffrin, Schneider and colleagues
provides a framework for understanding the important functional
implications of automaticity (Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977).
This framework was developed to explain two complementary forms of
cognitive processing, automatic and controlled, but the concepts can
also be applied to locomotor control. These researchers defined
automatic cognitive processing as the activation of a sequence of nodes
that nearly always becomes active in response to a particular input
configuration and that is activated automatically without the necessity
for attention by the individual (Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977).
Although the neural structures/networks underlying automatic cognitive
processing differ from those underlying automatic locomotor control (see
Section Neurophysiology of Automaticity), the two can be viewed as
conceptually analogous. The opposite of automatic processing is
controlled cognitive processing, which was defined as a temporary
sequence of nodes activated under control of, and through attention by,
the individual (Schneider and Shiffrin, 1977).
Controlled processes are capacity limited, but the costs of this
capacity limitation are balanced by the benefit of being set up,
altered, and applied in novel situations for which automatic sequences
have never been learned (Schneider and Shiffrin, 1977).
In the present paper on locomotor control, the broader term “executive
control” is used in place of “controlled processing”. There are a number
of important phenomena that characterize the difference between
automatic and controlled processing and that should be considered in the
context of walking performance and safety. The first phenomenon is that
automatic processing is fast and parallel, while controlled/executive
processing is slow and serial (Schneider and Chein, 2003).
In the context of walking, the use of an executive control strategy is
concerning because it is less suited for managing the complexities of
multi-joint movements in real time. For instance, crucial information
from the periphery, such as unexpected changes in the slope or texture
of the walking surface, must be quickly and accurately integrated into
the ongoing gait cycle for safe ambulation. With automaticity of
control, this information can be quickly delivered and integrated via
spinal reflex pathways (i.e., fast, parallel processing of information) (Zehr and Stein, 1999; af Klint et al., 2008).
In contrast, an executive control strategy would require a much longer
time period for peripheral information to be delivered and processed in
the cerebrum before subsequent integration with the gait pattern (i.e.,
slow, serial processing of information). Furthermore, the resultant
neural commands may be less appropriate and more variable. A second
phenomenon is that automatic processing requires little effort and can
operate in high workload situations, whereas controlled/executive
processing requires substantial effort and interferes with other
controlled processing tasks (Schneider and Chein, 2003).
This is concerning for walking because loss of automaticity and a
compensatory reliance on executive control could overly encumber the
available supply of executive resources. This will lead to a competition
for executive resources and may result in performance decrements for
walking and concurrent tasks (Ojha et al., 2009; Clark et al., 2014b).
Such a decrement is commonly referred to as the “cost” of
multi-tasking. This issue has also been described as a “supply and
demand problem”, such that the cumulative demand for executive control
resources exceeds the available supply (Seidler et al., 2010). A sufficient supply of executive resources is important for walking performance under complex environmental conditions (Clark et al., 2014b).
For example consider the demands of walking in a crowded shopping mall.
If the executive resources needed for this task are encumbered by the
control of the basic walking pattern, there is a heightened risk that
hazards may be overlooked or ignored. The individual may be less likely
to notice a slick puddle on the floor or may misjudge the speed or
direction of surrounding pedestrians, resulting in slips, trips,
collisions and falls. A third phenomenon is that automatic processing is
far less sensitive to stressors than is performance under
controlled/executive processing (Schneider and Chein, 2003).
This implies that environmental conditions that are challenging or
anxiety-provoking may substantially deteriorate performance of executive
locomotor control. One example is the challenge/anxiety associated with
walking across a busy street. Dommes and colleagues suggest that the
attentional demands of gait and balance in older adults may contribute
to instances of poor decision making and dangerous behaviors during
simulated street crossing. Specifically, older participants were found
to cross more slowly, adopt smaller safety margins, and make more
decisions that led to collisions than did young participants (Dommes et al., 2014).
The cumulative evidence indicates that compromised automaticity of
walking has important functional implications, which highlights the
crucial need for improved mechanistic understanding and enhanced
rehabilitative strategies.
Neurophysiology of Automaticity
Automaticity of walking is made possible by specialized
circuits in the central nervous system (CNS) that are capable of
coordinating complex patterns of neuromuscular activation. The circuits
have been fine-tuned over millions of years of evolution (Nielsen, 2003)
to allow for a stable yet flexible locomotor control strategy that does
not require continuous attentional control. The most well-described
circuits (primarily revealed by animal studies of locomotor control) are
located in the spinal cord, brainstem and cerebellum. This section will
briefly describe some of these major neural circuits.
The “central pattern generator” circuits of the spinal
cord are perhaps the most well-known locomotor circuits supporting
automaticity. Evidence from animals and humans reveals that
non-patterned electrical input to the lumbar spinal cord can elicit
flexion/extension movements of the limbs that are similar to walking,
even in the absence of input from the brain (Grillner, 1981).
For instance, Dimitrijevic and colleagues used epidural stimulation of
the posterior spinal cord to elicit locomotor-like limb movements in
adults with complete spinal cord injury. This finding complements
earlier research that demonstrated the ability of decerebrate cats to
perform basic stepping movements (Sherrington, 1910; Brown, 1911).
Spinal pattern generating circuits may already be operational at birth,
as they have been proposed to be responsible for coordinated kicking
movements in human infants, as well as the “step reflex” that occurs
when infants are stood upright with body weight supported (Forssberg, 1985). With maturation and practice, these circuits become more complex in order to facilitate coordinated adult locomotion (Ivanenko et al., 2004; Clark et al., 2010; Dominici et al., 2011).
At the next level of the neuraxis are brainstem circuits of locomotor
control. Electrical stimulation of isolated brainstem regions has been
shown to evoke walking-like behaviors. The two key regions that have
been identified are the mesencephalic locomotor region (MLR) and
subthalamic locomotor region (SLR). The MLR has been observed in all
vertebrate species tested to date, including lamprey, salamander,
stingray, rat, guinea-pig, rabbit, cat, and monkey (Le Ray et al., 2011; Ryczko and Dubuc, 2013).
It provides excitatory input to the spinal cord that serves to
initiate, scale, and sustain the descending command for walking (Le Ray et al., 2011; Ryczko and Dubuc, 2013).
The SLR is considered to be closely related to the MLR and has been
found in a number of vertebrates including rats and cats (Kasicki et al., 1991; Narita et al., 2002).
It may have particular relevance for scaling locomotor output, such as
when inducing changes in speed and cadence (e.g., walking vs. running) (Narita et al., 2002). In addition to brainstem locomotor regions, a cerebellar locomotor region has been reported in cats (Mori et al., 1998).
Furthermore, studies in humans with cerebellar damage have shown the
important role of the cerebellum in the control and coordination of
balance and walking (Morton and Bastian, 2004).
Among the notable findings with cerebellar damage are ataxic gait,
impaired motor learning, and compromised ability to make predictive gait
and balance modifications (Horak and Diener, 1994; Morton and Bastian, 2004, 2006).
Finally, descending excitatory drive from cerebral motor pathways is
considered crucial to facilitating the brainstem and spinal circuits of
automaticity in humans (Yang and Gorassini, 2006).
Emerging evidence from studies using electroencephalography and
transcranial magnetic stimulation further suggest a direct involvement
of motor cortex in driving muscle activation, even during undemanding
steady state walking (Petersen et al., 2001, 2012).
Accordingly, some aspects of automaticity of walking may reside in
cerebral circuits. Cumulatively, the CNS circuits discussed here
comprise the neurophysiological architecture that allows for
automaticity of walking without the need for continuous attentional
monitoring and executive control.
Measuring the Balance Between Automatic and Executive Control of Walking
A challenge to studying automaticity is that the CNS
circuits cannot be directly assessed in humans. Rather, the balance
between automaticity and executive control must be inferred from
assessments that gauge heightened utilization of an executive control
strategy during walking. The underlying premise is that, during
undemanding steady state walking, executive control is used as a
compensatory control strategy in the absence of robust automaticity.
Executive control involves the use of attentional and intentional
resources in the cerebrum to monitor and execute movements. The most
widely used approach for probing automatic vs. executive control is
assessment of dual-tasking. This is a behavioral approach in which a
single task of interest, such as walking, is performed alone
(single-task) as well as simultaneously with another task (dual-task).
Often, the dual-task condition yields a decrement in performance
compared to the single task condition. The size of the decrement, called
the “dual-task cost”, is interpreted to result from a competition for
executive control resources. When the single task requires heightened
executive control, the dual-task cost is expected to be higher. In
contrast, automatic control of the single task is expected to yield a
lower dual-task cost. Although the premise is fairly simple, in reality
the determinants of dual-task cost are multi-factorial and potentially
complicated. The instructions given to the participant, particularly
with regarding to task prioritization, are known to substantially
influence the results. Furthermore, the difficulty level of the
secondary task (often verbal fluency or mathematical problem solving)
varies greatly based on the task chosen and the capabilities of the
individual being tested. Prior articles have provided substantial
discussion and review of dual-tasking assessments (Beauchet and Berrut, 2006; Beauchet et al., 2009; Plummer et al., 2013; Patel and Bhatt, 2014).
Neurophysiological assessments offer an alternative
approach to dual-tasking for measuring the balance between automaticity
and executive control of walking. Among the most promising is functional
near infrared spectroscopy (fNIRS), because it provides continuous,
noninvasive, unobtrusive monitoring and can be used in ecologically
valid settings including during walking (Ayaz et al., 2012b; Holtzer et al., 2014; Perrey, 2014; Piper et al., 2014).
The major drawback of fNIRS is that it is limited to superficial
recording of cortex and has lower spatial resolution than functional
magnetic resonance imaging. During fNIRS assessment, a laser diode at
the surface of the skin emits near-infrared light which passes through
soft tissue and bone to reach the cerebral cortex. In the cortex, some
near-infrared light is absorbed by hemoglobin while a proportion of the
non-absorbed light scatters back to the surface. This non-absorbed light
is then measured by a highly sensitive photodiode. Because
oxyhemoglobin and deoxyhemoglobin preferentially absorb light of
different wavelengths, the concentration changes of oxy- and
deoxy-hemoglobin can be calculated. Hemoglobin concentrations are
directly affected by metabolic activity in cortical tissue and the
resultant changes in blood flow. In addition to fNIRS, other assessment
approaches also show great promise, including positron emission
tomography, electroencephalography, frequency-based analysis of
electrophysiological signals, and fMRI during imagined walking (Duckrow et al., 1999; Cham et al., 2008; Shoushtarian et al., 2011; Petersen et al., 2012; Clark et al., 2013; Shimada et al., 2013; Holtzer et al., 2014). Most of the relevant literature that is cited in the present article uses fNIRS as the neurophysiological assessment.
For monitoring the use of executive control resources,
an important brain region is prefrontal cortex. The prefrontal cortex
operates at the highest levels of the control hierarchy, contributing to
a cascade of processes that mediate task planning and execution for
cognitive and motor functions (Koechlin et al., 2003; Parasuraman and Caggiano, 2005; Bear et al., 2007). It plays an essential role as an interface between cognition, action, and the physical world (Derosière et al., 2013). The literature reports that prefrontal cortical activity is heightened during the performance of cognitive tasks (Herrmann et al., 2006; Kaneko et al., 2011; Ohsugi et al., 2013), fine motor tasks (Okamoto et al., 2004), and dual-tasks (Holtzer et al., 2011; Doi et al., 2013; Ohsugi et al., 2013). A number of studies have detected incremental increases in parallel with the complexity of cognitive tasks (Shibuya-Tayoshi et al., 2007; Kaneko et al., 2011; Ayaz et al., 2012b; Verner et al., 2013).
Likewise, a number of fNIRS studies of walking have demonstrated that
more complex walking tasks also require heightened prefrontal activity
relative to undemanding steady state walking. For example, prefrontal
activity is significantly elevated when preparing for gait initiation or
executing speed changes (Suzuki et al., 2004, 2008; Mihara et al., 2007; Clark et al., 2014b), during the performance of complex walking tasks that require careful control of posture and of limb movements (Atsumori et al., 2010; Clark et al., 2014b; Koenraadt et al., 2014), and during dual-task walking (Clark et al., 2014b; Meester et al., 2014).
In contrast, walking at different steady state walking speeds (e.g.,
slow speed vs. moderate speed) does not substantially affect prefrontal
activation (Suzuki et al., 2004; Meester et al., 2014).
This latter finding is presumably due to the ability of brainstem and
spinal circuits of automaticity to alter the rate of locomotor pattern
generation without the need for substantial executive control resources.
Heightened prefrontal activity may also reflect an increased executive
demand to compensate for loss of automaticity due to neurological or
peripheral (e.g., musculoskeletal) impairments (Seidler et al., 2010)
or due to impairment of CNS circuits of automaticity. Indeed,
prefrontal activity has been shown to be elevated during steady state
walking in the elderly, especially older adults with poorer gait
performance (Harada et al., 2009) and those with ataxic gait (Mihara et al., 2007; Caliandro et al., 2012).
Prefrontal fNIRS assessment has also shown potential for
explaining differences in functional task performance, including over
the course of motor learning during acquisition of automaticity for a
novel task. Ayaz and colleagues provide an excellent example of a
transition from executive to automatic control with task practice. They
measured the behavioral and neurophysiological responses of novice
participants while they practiced the complex cognitive/motor task of
piloting a virtual unmanned aerial vehicle in a flight simulator (Ayaz et al., 2012a,b).
With practice, all measures indicated improvement in performance when
comparing across beginner, intermediate and advanced phases of training.
Analysis of change across days revealed that behavioral measures of
flight performance provided more detailed information than subjective
self-reported measures. Furthermore, changes in prefrontal activation
measured by fNIRS provided even more detail. Specifically, at the
beginner level, behavioral performance improved each day at the cost of
heightened prefrontal activation (i.e., heightened executive demand).
This suggests that increased effort was required to learn the skill. In
the intermediate phase, a higher level of behavioral performance could
be maintained with less prefrontal activation. Finally, in the advanced
phase, an even higher level of behavioral performance was achieved with a
trend toward a further reduction of prefrontal activation (Ayaz et al., 2012a). It is reasonable to expect that similar acquisition of automatic control could be attained with rehabilitation of walking.
Also supporting the use of prefrontal fNIRS to gauge the
link between function and automaticity is work by Harada and
colleagues, who compared prefrontal activity and driving performance in
less experienced vs. more experienced drivers (Harada et al., 2007).
Young adults who are less experienced exhibited a larger increase in
prefrontal activity during driving, suggesting less automaticity. These
less experienced drivers also exhibited unsafe driving behaviors such as
not observing the door mirror carefully when changing lanes. This
observation may be indicative of competing demand for executive control
resources. Little research has been done thus far to link prefrontal
activity to walking performance (Clark et al., 2014b), so this will be an important area for future research.
The interpretation of prefrontal activity should take
into account the potential confounding effects of underlying
physiological factors. For instance, fNIRS activity has been shown to be
affected by the volume of underlying gray matter (Maillet and Rajah, 2013) and the health of the cerebrovascular and cardiovascular systems (Suhr and Chelberg, 2013).
These factors are often compromised in older adults, for example.
Similarly, individuals who are disengaged or unmotivated during
performance of experimental tasks may exhibit smaller changes in
cortical activity due to lower utilization of executive resources.
Furthermore, some research has reported that prefrontal fNIRS may lack
the sensitivity for detecting subtle changes in executive control (Derosière et al., 2013).
Although much of the existing research that has used fNIRS during
walking has assessed prefrontal cortex, it should be acknowledged that
this is not the only cortical region that is likely to be involved in
executive locomotor control. Indeed, prior fNIRS studies have shown
walking-related changes in motor and somatosensory cortical regions (Suzuki et al., 2004, 2008; Kurz et al., 2012; Koenraadt et al., 2014). It will be important for future research to assess a broader array of cortical regions.
Mechanistic Factors That Influence Automaticity
In the section entitled Neurophysiology of Automaticity,
the neural circuits supporting automaticity of walking were discussed.
This section will further expand upon mechanistic factors that may
compromise automaticity by influencing the operation of those circuits,
either directly or indirectly. The factors discussed include CNS
injury/disease, proprioception, tactile somatosensation, visual
impairment, physical effort, pain, state anxiety, use of assistive
devices, biomechanical structure and hearing impairment (Figure 1).
This should not be considered a definitive or all-inclusive list, but
rather focuses on factors that are potentially important to clinical
populations with compromised walking function. The order in which each
factor is presented is roughly based on the strength of evidence
supporting an effect on circuits of automaticity or on the balance
between automatic and executive control of walking.
FIGURE 1
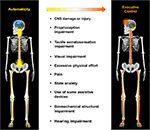 Figure 1. Mechanistic factors that compromise automaticity of walking.
A variety of factors may contribute to a shift in the balance of
locomotor control from automaticity to executive control. These include,
but are not limited to central nervous system (CNS) damage/injury,
proprioception impairment, tactile somatosensation impairment, visual
impairment, excessive physical effort, pain, state anxiety, use of some
assistive devices, biomechanical structural impairment and hearing
impairment.
Figure 1. Mechanistic factors that compromise automaticity of walking.
A variety of factors may contribute to a shift in the balance of
locomotor control from automaticity to executive control. These include,
but are not limited to central nervous system (CNS) damage/injury,
proprioception impairment, tactile somatosensation impairment, visual
impairment, excessive physical effort, pain, state anxiety, use of some
assistive devices, biomechanical structural impairment and hearing
impairment.Nervous System Damage/Injury
Damage or disease of the CNS can be devastating to
walking function, as demonstrated by conditions such as stroke, spinal
cord injury, Parkinson’s disease and others. The effect of CNS damage on
automaticity may be due to a number of different factors. One is direct
damage to CNS circuits of automaticity, such as may occur with injury
to the lumbar spinal cord, or stroke affecting the brainstem. A second
factor is disruption of facilitatory drive to circuits of automaticity,
such as due to cortical stroke or injury to the upper spinal cord. A
third factor is impairment of peripheral nervous system structure or
function, such as sensory inputs like proprioception or vision. These
and other impairments affecting automaticity are discussed below. The
implications for automaticity by damage to any particular structure and
its associated pathways will depend on the specific case. Walking
assessments of people with CNS deficits are generally consistent with
impairment of automaticity and heightened executive locomotor control,
including poor dual task performance and heightened activity of
prefrontal cortex during walking (Hyndman et al., 2006; Mihara et al., 2007; Dennis et al., 2009; Plotnik et al., 2009; Caliandro et al., 2012; Plummer-D’Amato and Altmann, 2012; Smulders et al., 2012; Panyakaew and Bhidayasiri, 2013).
Proprioception
Proprioception provides input to the CNS about limb
position and weight bearing, and is a crucial input for automaticity.
Muscle spindles and Golgi tendon organs are proprioceptive sensory
receptors that supply feedback about muscle length and musculotendinous
force, respectively (Prochazka, 1981; Jami, 1992).
This information plays an important role in triggering the initiation
and maintenance of muscle activity that produce key events in the gait
cycle (Dietz, 1996; Pearson, 2008).
For instance, proprioceptive information induced by treadmill movement
is sufficient for producing coordinated locomotor movements, even in
decerebrate cats that lack descending control from the brain (Dimitrijevic et al., 1998; Grillner et al., 2008).
Furthermore, removal of proprioceptive input by deafferentation reduces
the magnitude of knee and ankle extensor muscle activity by
approximately 70% (Hiebert and Pearson, 1999).
Proprioceptive information from the hip joint and associated
musculature is known to be important for appropriate control of gait
biomechanics in humans and animals (Andersson and Grillner, 1981; Dietz et al., 2002). Hip extension during late stance phase of walking contributes to the initiation of swing phase (Hiebert et al., 1996; McVea et al., 2005), and mechanical perturbation of the limb during swing phase alters hip flexor activity (Lam and Pearson, 2001).
Abnormal proprioceptive input to the CNS, such as due to impaired
proprioception and/or abnormal walking patterns (e.g., poor hip
kinematics which is common in clinical populations (Lee et al., 2005; Svehlik et al., 2009; Hyngstrom et al., 2010)), may significantly compromise automaticity of walking.
Tactile Somatosensation
Sense of touch and vibration in the lower extremities is
known to be a crucial factor that interacts with the central circuits of
automaticity. Fallon and colleagues reported that information from skin
mechanoreceptors on the sole of the foot exert a strong facilitation of
spinal motorneuronal activity in the lower limbs (Fallon et al., 2005).
Furthermore, cutaneous stimulation during walking in animals and humans
has been shown to induce phase-specific modulation of limb movements (Frigon and Rossignol, 2006).
Clinical research has consistently shown that decrements in tactile
perception are strongly associated with compromised performance on tests
of walking and balance (Resnick et al., 2000; Mold et al., 2004; Deshpande et al., 2008; Buchman et al., 2009; Cruz-Almeida et al., 2014).
Although loss of automaticity cannot be directly implicated in this
association, an interesting study by Paul and colleagues (Paul et al., 2009)
provides some support for such an assertion. They show that
dual-tasking ability is more impaired in older adult diabetics with
peripheral neuropathy compared to older adult diabetics without
peripheral neuropathy (Paul et al., 2009).
This finding implies that peripheral impairments necessitate an
increased demand and competition for executive control of walking,
consistent with a lack of automaticity. If impaired tactile perception
compromises automaticity, can augmenting tactile input enhance
automaticity? This question was recently examined in research conducted
by Clark and colleagues, who found that wearing textured insoles can
reduce prefrontal cortical activation during walking in older adults
with mild somatosensory deficits (Clark et al., 2014a).
Less prefrontal activity implies a lower demand for executive control
and thus a more automatic strategy of locomotor control. Furthermore,
this finding offers a potential mechanistic explanation (i.e., enhanced
automaticity) for numerous prior observations of improved static and
dynamic balance when wearing textured or vibrating insoles (Priplata et al., 2003; Palluel et al., 2008, 2009; Qiu et al., 2012; Lipsitz et al., 2015).
Visual Impairment
Visual information is a crucial sensory input that
facilitates safe walking. A number of studies have shown associations
between diminished or abnormal visual input and decrements in walking
performance, including during control of steady state walking (Helbostad et al., 2009; Swenor et al., 2014) and during more complex tasks like obstacle crossing and curb negotiation (Alexander et al., 2014a,b; Novak and Deshpande, 2014).
This may be due in part to impaired automaticity of walking. In one
study it was shown that reduced visual input due to dim lighting yields
an increase in prefrontal cortical activity during steady state walking,
suggesting a shift in the balance from automatic to executive control (Clark et al., 2014b). Similarly, dual-task cost in Parkinson’s patients was found to be exacerbated by walking in dim lighting (Pieruccini-Faria et al., 2014).
It has also been reported that the magnitude of mental effort required
for mobility covaries with the severity of visual impairment in patients
with retinitis pigmentosa (Turano et al., 1998). Accordingly, lack of visual information may be an important factor leading to compromised automaticity of walking.
Physical Effort
Evidence suggests a shift in the balance from
automaticity to executive control for tasks requiring higher levels of
physical effort (Bhambhani et al., 2006; Mandrick et al., 2013; Derosière et al., 2014). Mandrick and colleagues evaluated force variability and cognitive task performance in a dual-tasking paradigm (Mandrick et al., 2013).
Healthy participants performed an isometric grip task at 15% and 30% of
maximal effort while simultaneously performing a mental arithmetic
task. Compared to the 15% condition, the 30% condition yielded greater
variability of grip force, poorer performance on the mental task, and
greater activity in the prefrontal cortex. The latter finding is also
consistent with other recent studies which demonstrated that prefrontal
activity increases in parallel with higher levels of force output (Bhambhani et al., 2006; Derosière et al., 2014).
There are at least two major conditions where the detrimental effects
of physical effort on automaticity may manifest as poorer mobility
function: weakness and obesity. Both weakness and obesity increase the
physical effort needed to perform mobility tasks (Hortobágyi et al., 2003; Bragge et al., 2014),
and both are common in clinical populations. Consistent with the
assertion that obesity increases the attentional demands of postural
control, Mignardot and colleagues showed that both postural sway and
auditory reaction time were worse during unipedal stance for obese vs.
non-obese participants (Mignardot et al., 2010). A search of the literature revealed no studies to date that have examined the effects of weakness on automaticity of walking.
Pain
Pain has been linked to mobility deficits (Karttunen et al., 2012; Demura et al., 2014), and may disrupt the automaticity of walking through a number of mechanisms. One is intentional avoidance of pain (de Gier et al., 2003)
in which an individual may consciously adjust his movements in order to
minimize the occurrence of pain. In the context of walking, this would
imply the use of an executive locomotor control strategy. Another
mechanism may be interference between the neural control pathways for
pain and automaticity. Prior studies have demonstrated that pain exerts
strong inhibitory influences on motor activity through spinal and
cerebral mechanisms (Le Pera et al., 2001; Farina et al., 2003; Don et al., 2008).
The functional implications of pain have been examined using dual-task
paradigms. Most of the existing research has been conducted on patients
with low back pain. A study by Sipko and colleagues examined the
influence of a hard vs. soft surface on postural control in patients
with low back pain. They report that patients with higher pain levels
exhibit deficits in the postural adaptability to surface compliance and
greater use of executive control for balance (Sipko and Kuczyński, 2012).
A number of studies have found that abnormal trunk and postural control
during walking or standing balance tasks in patients with low back pain
is further compromised by the addition of a cognitive task (Lamoth et al., 2008; Sherafat et al., 2014).
In contrast, others have reported that postural sway and trunk
stiffness for a seated postural control task in patients with low back
pain were improved (Van Daele et al., 2010)
by the addition of a cognitive task. A possible reason for these
apparently discrepant findings may be the difficulty level of the
coordination task (Van Daele et al., 2010; Sherafat et al., 2014).
Additional research is needed to better understand the link between
pain and automaticity during walking, including in conditions other than
low back pain.
State Anxiety
Anxiety can increase the attention that is dedicated to
locomotor control, which implies a shift from automaticity to an
executive control strategy (Gage et al., 2003).
One form of anxiety that is applicable to walking is the fear of
falling, which is common in neurologically compromised and elderly
individuals. Heightened anxiety due to the fear of falling has been
linked to abnormal performance on tasks of balance and gait (Adkin et al., 2002; Brown et al., 2002; Carpenter et al., 2004; Hadjistavropoulos et al., 2012). For instance, Brown and colleagues compared walking performance on the ground vs. on an elevated walkway (Brown et al., 2002).
The elevated walking condition had a variety of effects on the walking
pattern including altered spatiotemporal variability, joint kinematics
and neuromuscular activation. Similarly, concerns about
self-presentation may pose another form of anxiety for individuals with
movement disorders. Self-presentation refers to a person’s attempt to
monitor and control how he is perceived by others (Leary, 1995).
A person with an impaired gait pattern who feels judged by others may
devote heightened attention to the control of walking in a conscious
attempt to move more normally. Preliminary evidence from Lamarche and
colleagues suggests that self-presentational concerns may be detrimental
to balance performance and fall risk (Lamarche et al., 2014).
Use of Assistive Devices
Assistive devices such as canes and walkers are vital for
facilitating independent functioning in individuals with a variety of
walking-related impairments. Improvements in walking ability with the
use of assistive devices can be due to reduced demand for limb loading
and improved balance/orientation due to somatosensory feedback from the
hands (Ely and Smidt, 1977; Bateni and Maki, 2005).
These benefits would be expected to improve the automaticity of
walking, based on the evidence reviewed earlier in this section. Yet
accumulating evidence suggests that the use of an assistive device can
actually increase the executive demands of walking due to the need to
control movement of the device in addition to movement of the limbs (Bateni and Maki, 2005).
Multiple studies have used dual-tasking paradigms and found that
walking with a cane or rolling walker slowed the performance of a
reaction time task (Wright and Kemp, 1992; Wellmon et al., 2006). In some cases, assistive devices may even contribute to the occurrence of injurious falls (Stevens et al., 2009).
These findings highlight the importance of appropriate selection and
customization of assistive devices for each patient, in order to
optimize physical assistance as well as automaticity of walking.
Biomechanical Structure
The biomechanical structure of the lower extremity,
including passive elastic properties of muscle and connective tissue,
are important to the coordination and efficiency of walking (Whittington et al., 2008; Zelik et al., 2014).
Research in the field of engineering has demonstrated that two legged
multi-jointed machines are capable of coordinated “walking” with little
to no source of external power (McGeer, 1993; Collins et al., 2005).
Although less complex than true human locomotion, these machines
demonstrate the impressive role of biomechanical features for producing a
well-organized pattern of walking. Therefore, factors that interfere
with these biomechanical features may be detrimental to locomotor
control. This could be particularly important in the context of clinical
populations who wear rigid braces or orthoses. A search of the
literature revealed a number of studies that have examined the link
between orthosis stiffness and aspects of gait performance (Bregman et al., 2011; Kobayashi et al., 2013; Harper et al., 2014),
but none that have directly tested the effect of biomechanical
constraints on the automaticity of walking. Although these supportive
devices serve an important role for the patient, advances in design and
materials may be advantageous if they restore or augment the natural
biomechanical features that contribute to control of walking (Takahashi and Stanhope, 2013).
Hearing Impairment
Hearing impairment has been shown to independently influence mobility function (Chen et al., 2014).
A direct link to automaticity has not yet been investigated but it is
reasonable to expect that such a link could exist. Auditory information
has been shown to be an important influence for modulating the steady
state walking pattern. For instance, a number of studies have used
rhythmic auditory stimulation as a means to alter the spatiotemporal
parameters of gait in patients with Parkinson’s disease, stroke, and
other neurological disorders (del Olmo and Cudeiro, 2005; Hausdorff et al., 2007; Kadivar et al., 2011; Wittwer et al., 2013; Rodger et al., 2014).
These studies generally report a positive influence, such as one by
Hausdorff and colleagues who reported a more automatic movement pattern
with less stride-to-stride variability when gait was timed to a
metronome (Hausdorff et al., 2007).
Furthermore, evidence suggests that auditory information is an
important factor causing unintentional synchronization of stepping when
humans walk side-by-side (Zivotofsky et al., 2012). Based on these findings, hearing impairment has the potential to compromise automaticity of walking.
More at link including Strategies for Rehabilitation.
 David J. Clark
David J. Clark
No comments:
Post a Comment