I can't see anything here that even remotely looks like a protocol on how to handle these dissections. So completely useless! If we had survivors in charge we would get protocols out of stroke research that gets survivors recovered!
Treatment and Outcomes of Cervical Artery Dissection in Adults: A Scientific Statement From the American Heart Association
Abstract
Cervical artery dissection is an important cause of stroke, particularly in young adults. Data conflict on the diagnostic evaluation and treatment of patients with suspected cervical artery dissection, leading to variability in practice. We aim to provide an overview of cervical artery dissection in the setting of minor or no reported mechanical trigger with a focus on summarizing the available evidence and providing suggestions on the diagnostic evaluation, treatment approaches, and outcomes. Writing group members drafted their sections using a literature search focused on publications between January 1, 1990, and December 31, 2022, and included randomized controlled trials, prospective and retrospective observational studies, meta-analyses, opinion papers, case series, and case reports. The writing group chair and vice chair compiled the manuscript and obtained writing group members’ approval. Cervical artery dissection occurs as a result of the interplay among risk factors, minor trauma, anatomic and congenital abnormalities, and genetic predisposition. The diagnosis can be challenging both clinically and radiologically. In patients with acute ischemic stroke attributable to cervical artery dissection, acute treatment strategies such as thrombolysis and mechanical thrombectomy are reasonable in otherwise eligible patients. We suggest that the antithrombotic therapy choice be individualized and continued for at least 3 to 6 months. The risk of recurrent dissection is low, and preventive measures may be considered early after the diagnosis and continued in high-risk patients. Ongoing longitudinal and population-based observational studies are needed to close the present gaps on preferred antithrombotic regimens considering clinical and radiographic prognosticators of cervical artery dissection.
Cervical artery (internal carotid or vertebral artery) dissection can occur in the absence of major trauma.1 It is usually attributable to an intimal tear or rupture of the vasa vasorum. This can lead to an intraluminal thrombus, vascular stenosis, occlusion, or dissecting aneurysm formation. Cervical artery dissections can present with local signs and symptoms (eg, pain or cranial nerve compression) or cerebral or spinal cord ischemia.1
Cervical artery dissection is an important cause of stroke and stroke-related disability in young adults.2 Despite high-quality studies, data conflict on the diagnostic evaluation, treatment, and outcomes of patients with suspected non–major trauma–associated cervical artery dissection with or without cerebral ischemia.2 Conflicting evidence has led to clinical equipoise and variability in practice across clinicians.3
In this scientific statement, we aim to provide an overview of non–major trauma–associated cervical artery dissection, hereafter referred to as cervical artery dissection, with a focus on summarizing the available evidence on the diagnostic evaluation, treatment approaches, and outcomes.
METHODS
A multidisciplinary team of 11 writing group members from neurology, neuroradiology, neurosurgery, emergency medicine, and interventional neurology were identified to cover the sections. Each writing group member wrote and reviewed at least 1 section. In this scoping review, authors performed a literature search using key words relevant to each section, and the search results were reviewed for relevant publications. The literature search focused on publications between January 1, 1990, and December 31, 2022, and included randomized controlled trials, prospective and retrospective observational studies, meta-analyses, opinion papers, case series, and case reports. Apart from meeting presentations for clinical trials, meeting abstracts were not included.
Using the information from relevant publications, the writing group members drafted their sections. Once the sections were complete, the writing group chair and vice chair compiled the manuscript and circulated it to the writing group members for review and feedback. Multiple drafts were circulated among writing group members until a consensus was achieved. A summary of suggestions to clinicians pertinent to each section is provided in the Table.
| Epidemiology | Cervical artery dissection is a common cause of stroke in younger adults and caused by the interplay between comorbidities, genetic or congenital factors, anatomic factors, and environmental triggers. | |
| Clinical diagnosis | It is reasonable to perform urgent cervical vascular imaging with CTA or MRI with MRA in patients with headache or neck pain associated with symptoms of ischemia or a partial Horner syndrome and considered in those with new or worsening headache or neck pain, especially when there is a history of minor cervical trauma or other risk factors for cervical artery dissection. | |
| Imaging tools for diagnosis | In patients with suspected cervical artery dissection, an MRA or CTA is a reasonable test to consider. In patients with negative CTA and continued clinical concern for cervical artery dissection, MRA with fat-suppressed images may be considered, given the high sensitivity to visualize a mural hematoma. DSA should be avoided as a first-line diagnostic tool but may be considered in patients with clinical concern and negative MRA and CTA. Ultrasound might be useful for follow-up assessments of arterial remodeling. | |
| Imaging diagnostic challenges | Imaging signs of dissection include a tapering stenosis or occlusion, intramural hematoma, dissecting aneurysm, double lumen, and dissection flap. It is crucial to carefully review the cervical vascular imaging studies of patients with ≥1 signs of dissection present and to recognize dissection mimics such as carotid artery web, fenestrated artery, pseudo-occlusion, and imaging artifacts. | |
| Role of genetic testing | It is reasonable to screen patients with cervical artery dissection for clinical signs of monogenic connective tissue disorders (eg, recurrent dissection, family history, physical examination) and to perform appropriate genetic counseling and testing based on the previously discussed screening tools. Routine genetic testing in the absence of signs or symptoms of monogenic connective tissue disorder is not suggested because of its increased cost and low yield but may be considered in patients with recurrent dissection. | |
| Diagnostic testing after dissection diagnosis | In patients with cervical artery dissection, it is reasonable to screen for cerebral aneurysm and aortic root dilation. In those with hypertension or evidence of fibromuscular dysplasia, screening for renal artery stenosis with a renovascular Doppler is reasonable. | |
| Timing and predictors of ischemic stroke | The early ischemic stroke risk supports the timely recognition, diagnosis, and initiation of optimal antithrombotic treatment for cervical artery dissection. Clinicians can use demographics, clinical characteristics, and imaging findings to predict which patients are at higher risk of developing an ischemic stroke after cervical artery dissection. | |
| IVT | In the absence of data suggesting safety concerns and given the proven efficacy of IVT in otherwise eligible patients with acute ischemic stroke, it is reasonable to consider IVT for patients with acute ischemic stroke with cervical artery dissection if they meet other standard criteria, as recommended by current guidelines. For patients with intracranial extension of the dissection, the risks and benefits of IVT are not well established. | |
| Mechanical thrombectomy | It is reasonable to perform mechanical thrombectomy in otherwise eligible patients with a large-vessel occlusion in the setting of cervical artery dissection. | |
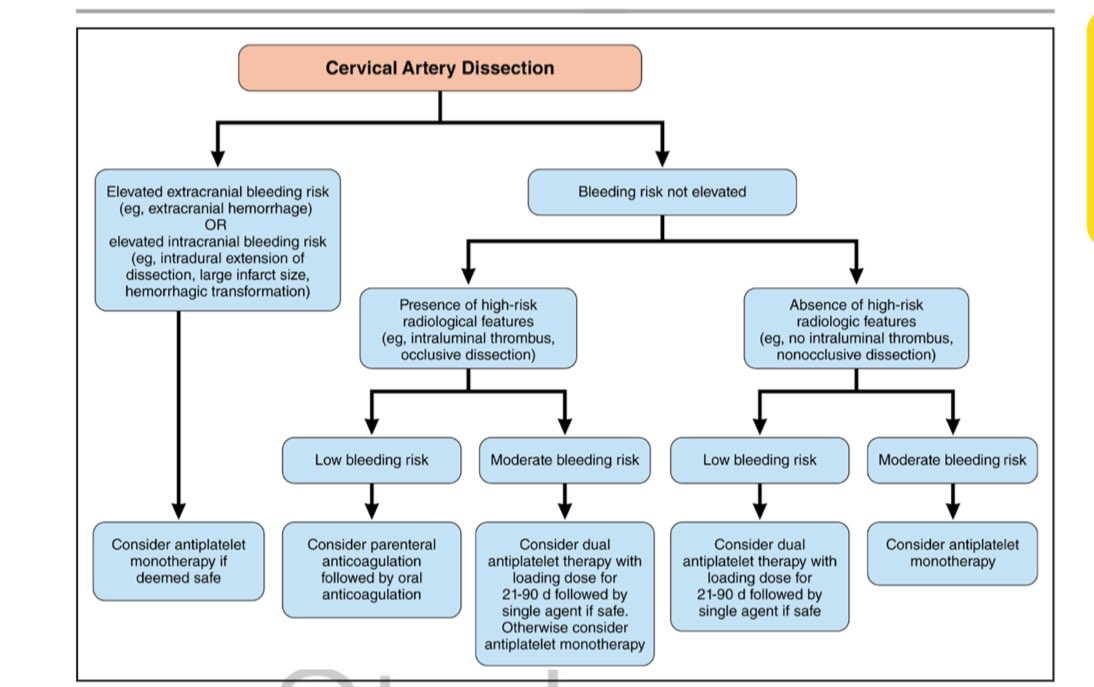
| Antithrombotic treatment | Because most ischemic events occur within the first several days after diagnosis, optimizing antithrombotic treatment (when safe) early is of paramount importance. In addition, it is reasonable that clinicians start immediate antithrombotic treatment if deemed safe. Parenteral followed by oral anticoagulation may be considered in particular in patients at low risk of intracranial hemorrhage (small infarct size, no intradural extension, and no intracranial hemorrhage) but at high risk for ischemic stroke (eg, intraluminal thrombus, occlusive dissection). Although the evidence for use in cervical artery dissection is weak, a short course of dual antiplatelet therapy with a loading dose (followed by single antiplatelet agent) might be preferred over monotherapy when deemed safe, particularly in patients who would have qualified for the dual antiplatelet trials for early prevention after minor stroke/TIA. Otherwise, antiplatelet monotherapy could be used. In the absence of a general superiority of any antithrombotic regimen, it is reasonable that clinicians individualize treatment on the basis of the risk of ischemic stroke and ICH, as well as a shared decision-making process with their patients. | |
| Antithrombotic treatment duration | It is reasonable that the duration of antithrombotic therapy in patients with cervical artery dissection be 3–6 mo. Decisions to extend antithrombotic therapy past the 6-mo mark may be considered in the context of an individual’s overall vascular risk factor profile and in the context of neuroimaging features as remodeling occurs. | |
| Subacute stenting | Patients with cervical artery dissection with significant stenosis causing distal hemodynamic compromise AND recurrent ischemic stroke despite optimal medical treatment AND who can withstand surgery may be considered for stenting as a measure for secondary stroke prevention. | |
| Risk and predictors of recurrent dissection | The risk of recurrent cervical artery dissection is 1%–2% per y but is particularly increased in the first few months after the initial cervical artery dissection. Younger age and fibromuscular dysplasia are risk factors for dissection recurrence. | |
| Precautions to reduce the risk of dissection recurrence | Because the risk of recurrent or worsening dissection is highest in the first few months after the initial dissection, it is reasonable that all patients with cervical artery dissection avoid activities that increase the risk of cervical injury for 1–6 mo from diagnosis and until healing of the index dissection. Furthermore, although there are no proven precautions to reduce the long-term risk of recurrent dissection, it is reasonable for health care clinicians to suggest that patients with cervical artery dissection who are at high risk for recurrent cervical dissection (eg, known connective disorder, recurrent dissection) avoid such activities lifelong. | |
| Radiological recanalization of the dissection | Recanalization of cervical artery dissections occurs mostly in the first 12 mo after diagnosis. Cervical artery–dissecting aneurysms infrequently increase in size, become symptomatic, and require treatment. Recanalization and development or resolution of cervical artery–dissecting aneurysms do not seem to be affected by antithrombotic treatment regimens. | |
EPIDEMIOLOGY/RISK FACTORS
Cervical artery dissection contributes to 2% of all ischemic strokes4 but up to 25% of ischemic stroke in adults <50 years of age.5,6 Prior studies reported an incidence rate of 2.6 to 3.0 per 100 000 people4,7,8 but the true incidence is likely higher,9 partly because some patients with dissection may not seek medical attention because of self-limited or minor symptoms.
The mean age at diagnosis is 45 years.7 Although the proportion of dissection-related stroke is higher in younger patients, the absolute prevalence of dissection-related ischemic strokes increases with age.10 The incidence is slightly higher in men,11 but the age at onset and the peak age prevalence of dissection-related stroke are lower in women (30–49 years) compared with men (50–89 years).10,11
The pathogenesis of cervical artery dissection is multifactorial and involves the interplay of comorbidities, environmental triggers, genetic or congenital factors, including connective tissue disorders, and anatomic factors such as elongated styloid process (>30 mm; Eagle syndrome) or increased vascular tortuosity (Figure S1).1,12–14 Furthermore, risk factors may differ according to dissection location; recent systemic infection is more common in carotid artery dissection, and history of minor trauma is more common in vertebral artery dissection.15
CHALLENGES IN DIAGNOSIS
Clinical Diagnostic Challenges
Because of the often nonspecific nature of symptoms (eg, headache, neck pain, dizziness, and tinnitus), diagnosis of cervical artery dissection is challenging, especially in the absence of ischemic or localizing symptoms. In a statewide study of claims data, 3.1% of patients with cervical artery dissection were previously seen in the emergency department for possible related symptoms in the 14 days preceding diagnosis16; younger and female patients were more likely to have a probable missed diagnosis.16
Evaluation for suspected cervical artery dissection should include a detailed symptom history, questions about minor trauma, and a detailed neurological examination. Common initial symptoms of cervical artery dissection include facial pain, headache (≈65%), and neck pain (≈50%),15 which tend to precede cerebrovascular ischemic symptoms17,18; thus, early diagnosis and treatment could reduce the risk of cerebrovascular ischemia. Isolated pain (without cerebral ischemia) occurs in 8% to 12% of diagnosed cases.19,20 For internal carotid dissections, pain is often facial, frontal, or temporal, whereas vertebral artery dissections generally cause pain in the cervical or occipital area.17,21 In carotid dissection, pain may also be associated with a (partial) Horner syndrome (≈25%7) and cranial nerve palsies (≈12%),22 and both carotid and vertebral dissection can be associated with pulsatile tinnitus (≈8%).17,23,24 In fact, a partial Horner syndrome in a patient with new or worsening headache may suggest a carotid dissection diagnosis. Headache characteristics are not specific but can rarely be acute and of thunderclap nature.
Diagnostic Imaging Tools and Their Yield
The diagnostic modalities for cervical artery dissection are magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA), computed tomography angiography (CTA), ultrasound, and conventional digital subtraction angiography (DSA). Historically, DSA has been considered the reference imaging technique to demonstrate the lumen content and can delineate the presence of an intimal flap, double lumen, and dissecting aneurysm.25 DSA, however, provides limited information on the arterial wall (ie, intramural hematoma). Moreover, DSA carries periprocedural risks (≈0.5% iatrogenic dissection26 and 0.15% stroke).27
CTA is an effective noninvasive alternative to DSA that can display luminal contour alterations with rapid acquisition time, ready availability, and high spatial resolution; disadvantages include the exposure to radiation and iodinated contrast, and for intramural hematoma, CTA may show an eccentric or crescent-shaped vessel wall thickening or the rind sign in vertebral dissection28 (Figure 1), but these findings are neither sensitive nor specific to cervical artery dissection. A false-positive CTA can be due to streak artifact mimicking a double lumen and pulsation artifact mimicking an intramural hematoma. A false-negative CTA could be seen in the setting of a non–flow-limiting nondominant vertebral artery dissection but most commonly is due to failure of attributing an abnormality to dissection.29

Figure 1. Imaging signs suggestive of dissection diagnosis. A, Double lumen. B, Tapered or flame-shaped occlusion at typical dissection site. C, Intimal flap. D, Pseudoaneurysm. E, Intramural hematoma on fat-suppressed T1. F, Carotid imaging duplex mural hematoma (white arrows) as hypoechogenic structure compromising the arterial blood flow in the vertebral artery (V3 segment). G, Axial source image from computed tomography angiography (CTA) neck in a patient with complete occlusion of the left internal carotid artery (ICA) related to a dissection, showing the ring sign, with enhancement of the carotid wall and fresh nonenhancing thrombus within the carotid lumen. This sign is nonspecific for ICA dissection but points to a recent rather than a chronic occlusion. H, Axial source image from CTA neck in a patient with left vertebral artery dissection, with thrombus surrounding the hypoplastic but apparently normal lumen vertebral artery, known as the suboccipital rind sign, involving the atlanto-occipital (V3) segment.
MRA may be superior to CTA for the identification of the intramural hematoma when the appropriate protocol including axial fat-suppressed T1-weighted images is used. Limitations of MRA include the cost, availability, patient-related restrictions (eg, pacemakers and claustrophobia), and diagnostic limitations of time-of-flight MRA of the neck (eg, signal loss due to turbulence or vessel turn, susceptibility to artifact, and flow reversal artifact). False-positive MRA can be due to a hyperintense signal in the setting of turbulent flow and adjacent structures simulating a periarterial double-lumen sign. False-negative MRA can be related to the absence of hyperintense signal of the intramural hematoma in the hyperacute setting.29
Overall, both MRA and CTA have good sensitivity and specificity for diagnosis of cervical arterial dissection. MRA has high sensitivity in diagnosing carotid dissection (95%), but its sensitivity is lower for vertebral artery dissection (60%) compared with DSA.30 This is not the case with CTA, which has been shown to have similar sensitivity and specificity compared with DSA in diagnosing vertebral artery dissection.25
Ultrasound with color Doppler is noninvasive but is operator dependent and is of poor diagnostic utility,31 especially when the dissection is high cervical. Ultrasound may be helpful in rare cases with hyperacute dissection where the intramural hematoma can be visualized on ultrasound but not MRA (Figure 1). Ultrasound generally requires confirmation by CTA or MRA. Ultrasound has been shown to be useful for follow-up assessments within the first 4 weeks, when arterial remodeling is most prevalent.32
No comments:
Post a Comment