Where is the protocol on this so survivors can train their 'professionals' in it?
Comparison of tendon and muscle belly vibratory stimulation in the treatment of post-stroke upper extremity spasticity: a retrospective observational pilot study
Scientific Reports volume 14, Article number: 4151 (2024)
Abstract
Previous studies have reported the effects of vibratory stimulation (VS) therapy in reducing upper extremity spasticity after stroke. However, the effective location of the VS in patients with stroke remains unclear. This study aimed to determine the VS location that is most effective in reducing post-stroke finger and wrist flexor spasticity. We enrolled 27 consecutive patients with stroke and upper extremity spasticity in this retrospective observational study. The participants received stretching, tendon vibration, and muscle belly vibration for 5 min over a period of 3 days. To evaluate spasticity, we assessed the Modified Ashworth Scale score before and immediately after each treatment and immediately after voluntary finger flexion. Participants who received tendon vibration showed greater improvement in flexor tone in the fingers than participants who received stretching and muscle belly vibration (P < 0.05 and < 0.001, respectively). Participants who underwent VS showed no significant improvement in the wrist flexor tone compared to those who underwent stretching. Our results suggest that the tendon may be the most effective location for treating spasticity of the finger flexor muscles and that VS may not significantly improve spasticity of the wrist flexors more than stretching.
Introduction
Spasticity is a movement disorder characterized by a velocity-dependent increase in muscle tone, with exacerbated tendon reflexes1. Upper extremity spasticity occurs in approximately 35% of patients within 6 months of stroke onset2. Severe spasticity is more common in the upper than in the lower extremities2. Stroke patients may experience joint contractures, muscle pain, and limitations in their daily activities due to spasticity. This spasticity can also become a barrier to improving upper extremity function3,4. This highlights the importance of treating spasticity to facilitate the improvement of upper extremity hemiparesis.
Although botulinum toxin injection5,6,7,8 and intrathecal baclofen therapy9 are well-tolerated and effective treatments for spasticity after stroke, these therapies require special clinical skills and are invasive procedures that may cause pain. Moreover, these therapies are usually not cost-effective10 due to their high cost. In contrast, stretching11, extracorporeal shock wave therapy12,13, neuromuscular electrical stimulation14, and vibratory stimulation (VS)15,16,17 serve as non-pharmacological and non-invasive alternatives for the treatment of spasticity.
The American Heart Association guidelines recommend that VS be considered a non-invasive and effective treatment for reducing spasticity18. Three randomized controlled trials reported that local muscle VS may be a useful tool with anti-spastic effects when applied directly to the spastic muscles of the hemiplegic upper extremity after stroke15,16,17. Of these studies, Noma et al.15 implemented VS on the tendon and Costantino et al.16 implemented VS on the muscle belly; both studies reported improvement in muscle tone as measured by the Modified Ashworth Scale (MAS). However, no previous studies have compared the effects of VS on the muscle belly and tendon on upper extremity spasticity in patients with stroke. Owing to a lack of knowledge, no consensus has been reached as to whether the muscle belly is a more effective location than the tendon in VS therapy for spasticity. A recent systematic review of the effectiveness of VS for spasticity in patients with stroke also suggested that treatment effectiveness may vary depending on the target muscles and the degree of spasticity19.
This study aimed to determine whether VS of the tendon or muscle belly is more effective in reducing spasticity of the finger and wrist flexors in patients following stroke. Investigating the most effective location may enhance the effectiveness of VS therapy for spasticity in patients with stroke.
Methods
Study design and research participants
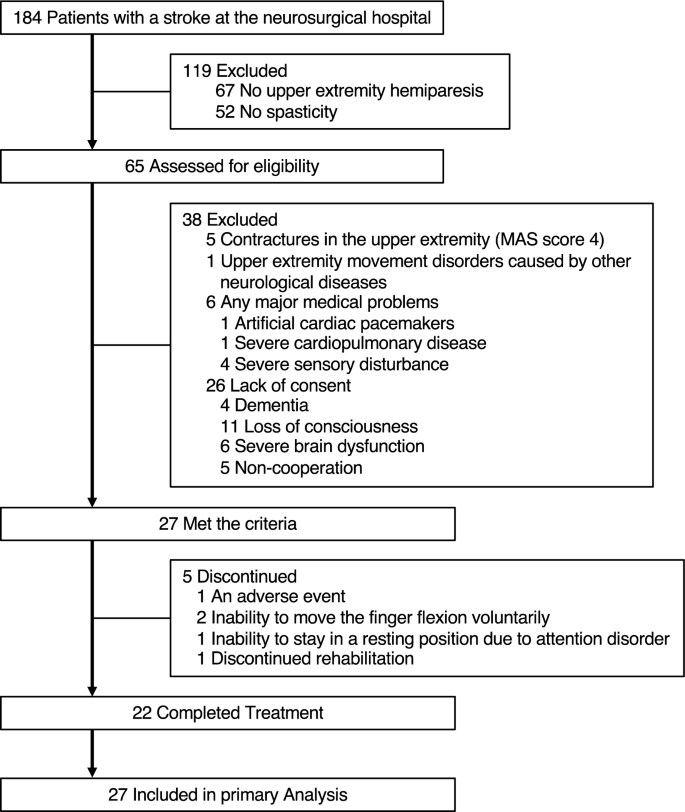
This retrospective observational study was conducted at our hospital. We recruited 27 consecutive patients with stroke who met the following criteria (Fig. 1): upper extremity hemiparesis, abnormal muscle tone of the affected wrist or finger flexors (MAS score 1–3); age > 20 years, and providing informed consent between November 2018 and March 2019.
The exclusion criteria were as follows: contractures in the upper extremity (MAS score 4), upper extremity movement disorders caused by other neurological diseases, and any major medical problems determined by the physician (artificial cardiac pacemakers, severe cardiopulmonary disease, or severe sensory disturbance).
The study was approved by the nonprofit organization MINS Institutional Review Board (acknowledgment number: 180229), and was designed according to the 1975 Declaration of Helsinki, as revised in 1983. The trial was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN000043457), which is a public trial registry.
Procedure
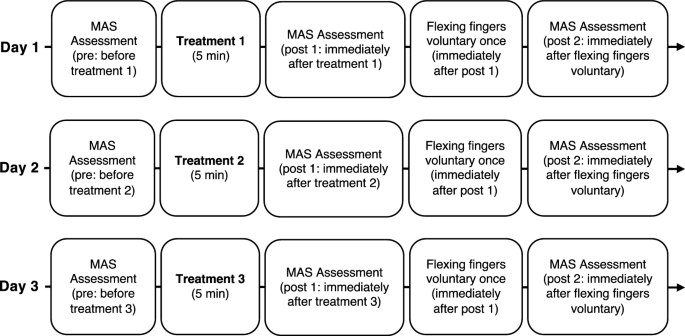
Figure 2 shows the typical procedure. The participants received three treatments once a day for 3 days. The order of treatment was randomized for each participant by the author. Occupational Therapist in charge of patient’s treatment performed the treatments and evaluations. The authors created a manual for the evaluation and treatment procedures based on previous studies15,20. We handed it out to the occupational therapists responsible for the evaluation and treatments. All occupational therapists underwent instruction in the methodology for assessing muscle tone through the Modified Ashworth Scale (MAS) and received training in three distinct therapeutic modalities under the author's guidance during practical skills development. The MAS scores were recorded three times for each treatment: before treatment (pre), immediately after treatment (post 1), and immediately after voluntary finger flexion (post 2).
Study design time flow. The order of the three treatments was randomized for each participant. Three treatments consisted of stretching for the wrist and finger flexor muscles, vibration stimulation therapy on the tendon of the wrist and finger flexor muscles, and vibration stimulation therapy on the muscle belly of the wrist and finger flexor muscles. MAS: Modified Ashworth Scale.
Participants flexed their fingers voluntarily immediately after recording the post 1 MAS scores, which were recorded again (post 2) to assess the lasting effect of reducing spasticity.
This study is not blinded in its evaluation, treatment, or analysis.
Interventions
The participants received the following three treatments: (1) stretching of the wrist and finger flexors, (2) VS therapy for the tendon of the wrist and finger flexors, and (3) VS therapy for the muscle belly of the wrist and finger flexors. The participants received each treatment for 5 min. During each treatment, the participants lay in the supine position and were asked to relax their muscles, as muscle contractions can interfere with the effects of the VS. If they could not receive the interventions in the supine position, they received the treatments in the sitting position.
In each treatment, the participant’s arm was placed in the maximum extension position using a specific device (Fig. 3A) to suppress the initial intense contraction by VS. The device had a movable wrist joint section that could be adjusted to fix the wrist joint in the maximum extension position. The device and treatment methods used were based on a previous study15. The device was developed by Teijin Pharma Ltd, Tokyo, Japan.
VS was applied to the tendon or muscle belly of the wrist and finger flexors using a vibration massager with spherical rubber and a vinyl-covered head (diameter: 5 cm) (Thrive MD-01; Thrive Co. Ltd., Osaka, Japan) (Fig. 3B,C). The vibration frequency was set to 91 Hz at an amplitude of 1.0 mm.
Measurement of muscle tone
The MAS20 was used to individually evaluate the spasticity of the wrist and finger flexors, and the scales rated the resistance perceived when moving an extremity passively about a joint in six grades (0, 1, 1+, 2, 3, and 4). A score of 0 indicates normal muscle tone, and 4 indicates rigid flexion or extension. MAS has mainly been used in previous studies to evaluate the spasticity of the biceps brachii, wrist flexors, and finger flexors20. For data analysis, MAS scores (0, 1, 1+, 2, 3, and 4) were assigned numerical values called “calculated MAS scores” (0, 1, 2, 3, 4, and 5, respectively)21.
We placed the joint in a maximally flexed position and moved it to a position of maximal extension for one second20.
The response rate was used to evaluate the response of spasticity to each treatment. This was defined as the proportion of participants with at least a 1-point improvement from baseline (pre) on the MAS.
Statistical analysis
No statistical sample size calculations were conducted. However, we conducted post-hoc power and effect size analyses using the results for the 27 participants in this study in GPower version 3.1. We calculated post-hoc power and effect size from the sum of the squares calculated in the analysis of variance.
This study included data from participants who did not complete the entire study process in the data analysis. Non-parametric statistics were used for the analyses because not all data met the normality criterion. This study did not compare patient characteristics between treatments because the three different treatments were administered to the same subjects. The effects of the interventions over time on the MAS scores were evaluated using 2-way repeated measures analysis of variance (ANOVA) with a post-hoc Bonferroni-corrected Wilcoxon test (number of comparisons = 3). Between-group differences in MAS scores were analyzed using the Kruskal–Wallis test and Bonferroni-corrected Wilcoxon test (number of comparisons = 3). To compare response rates between interventions, McNemar’s test was conducted. The threshold for significance was set at P < 0.05. All statistical analyses were performed using JMP version 17.0 software (SAS Institute Japan, Tokyo, Japan).
Results
Table 1 provides participants’ demographic information. The number of participants included in the analysis for each treatment is shown at the bottom of Figs. 4 and 5. One participant presented with blushing, a hot feeling, and swelling as adverse events after VS of the muscle belly. However, the symptoms improved the following day. Five participants received partial treatment because of an adverse event (N = 1), inability to move the finger flexion voluntarily (N = 2), inability to stay in a resting position due to attention disorder (N = 1), and discontinued rehabilitation (N = 1).
More at link.

No comments:
Post a Comment