So still NO PROTOCOL on oxygen delivery.
I can't see any use for HBOT unless it's delivered in the first week and there are vastly easier options for delivering oxygen than that.
oxygen delivery (29 posts to January 2020) Many ideas in here, if your doctor isn't already using them to save neurons immediately post stroke; you don't have a functioning stroke doctor!
Research progress on high-concentration oxygen therapy after cerebral hemorrhage
- 1Department of Neurology, Clinical Medical School of Jiujiang University, Jiujiang, Jiangxi, China
- 2Jiujiang Clinical Precision Medicine Research Center, Jiujiang, Jiangxi, China
- 3Department of Anorectal Surgery, Third Affiliated Hospital of Wenzhou Medical University, Zhejiang, China
Recently, the role of high-concentration oxygen therapy in cerebral hemorrhage has been extensively discussed. This review describes the research progress in high-concentration oxygen therapy after cerebral hemorrhage. High-concentration oxygen therapy can be classified into two treatment methods: hyperbaric and normobaric high-concentration oxygen therapy. Several studies have reported that high-concentration oxygen therapy uses the pathological mechanisms of secondary ischemia and hypoxia after cerebral hemorrhage as an entry point to improve cerebral oxygenation, metabolic rate, cerebral edema, intracranial pressure, and oxidative stress. We also elucidate the mechanisms by which molecules such as Hypoxia-inducible factor 1-alpha (HIF-1α), vascular endothelial growth factor, and erythropoietin (EPO) may play a role in oxygen therapy. Although people are concerned about the toxicity of hyperoxia, combined with relevant literature, the evidence discussed in this article suggests that as long as the duration, concentration, pressure, and treatment interval of patients with cerebral hemorrhage are properly understood and oxygen is administered within the treatment window, it can be effective to avoid hyperoxic oxygen toxicity. Combined with the latest research, we believe that high-concentration oxygen therapy plays an important positive role in injuries and outcomes after cerebral hemorrhage, and we recommend expanding the use of normal-pressure high-concentration oxygen therapy for cerebral hemorrhage.
1 Introduction
Spontaneous intracerebral hemorrhage (ICH) refers to the hemorrhage of brain parenchyma caused by vascular rupture caused by non-traumatic causes. With an increase in population age and widespread use of antithrombotic drugs, risk factors such as hypertension, diabetes, obesity, and alcohol abuse have increased, and the incidence of ICH is also increasing (1). Its high mortality and disability rates are closely related to neuronal damage caused by pathological reactions such as perifocal hypoxia after ICH. Surviving patients often experience permanent sequelae (2).
Brain injury after ICH can be classified into primary and secondary injuries. The primary injury is mechanical compression and expansion of the hematoma, which are key factors in determining the progression and outcome of ICH. They are generally caused by continued bleeding from ruptured blood vessels (3), usually occurring within 6 h after ICH, which can induce a space-occupying effect, compress blood vessels, reduce the volume of the vascular bed, increase intracranial pressure (ICP), decrease local perfusion, inhibit membrane ion pump activity (4), increase intracellular sodium ions, decrease intracellular crystal osmotic pressure, and form cytotoxic edema (5). The decrease of calcium ions in serum impairs the thrombin cascade reaction, and coagulation dysfunction (3) forms thrombosis, resulting in cerebral microcirculation obstruction and decreased oxygenation.
Hematoma components can induce secondary ICH. Thrombin is one of the components of hematoma. A high concentration of thrombin activated by the thrombin cascade reaction after ICH is closely associated with secondary ICH injury (4, 6). Thrombin can induce the production of inflammatory mediators, cause nerve cell damage (7), and influence neurological outcomes after ICH. Thrombin can also cleave the protease-activated receptors (PARs) receptor in microglia, which in turn phosphorylates the Src family kinase, thereby aggravating brain edema and the destruction of the blood–brain barrier. Stimulated microglia can also destroy tight junction proteins by up-regulating the expression of tumor necrosis factor (TNF) (6), increasing blood–brain barrier permeability. The complement cascade activated by thrombin may promote inflammation and edema after ICH through anaphylactoid toxins, or it may lyse red blood cells through membrane complexes to produce hemoglobin, iron, and oxygen free radicals, promote edema and oxidative stress and accelerate apoptosis after ICH, death, and blood–brain barrier disruption (4, 6). Red blood cells are also one of the components of hematoma. After intracerebral hemorrhage, the blood overflows from the ruptured blood vessels, and some red blood cells that are not completely phagocytized by microglia and infiltrating macrophages are directly released into the central system. Substances such as hemoglobin, iron, and carbonic anhydrase-1 are neurotoxic and are closely related to secondary brain injury after ICH (8). In addition, the automatic adjustment function of cerebral blood flow is based on a formula for cerebral blood flow, cerebral perfusion pressure (CPP), and cerebral vascular resistance (CVR). CVR can be adjusted to ensure the supply of cerebral blood flow (CBF) when ICP increases and CPP decreases. Expanded hematoma and high intracranial pressure after ICH cause decompensation of this regulation, further reducing CPP and aggravating cerebral ischemia (9). It is important to note that because hypertension is the most common cause of ICH (10), antihypertensive measures are often taken to alleviate the expansion of ICH hematoma; however, this is undoubtedly a challenge to the automatic regulation of cerebral blood flow. Therefore, attention should be paid to the tolerance of patients with hypertensive ICH to rapid blood pressure reduction; otherwise, insufficient cerebral perfusion will occur (11). Adnan et al. recommended that for intensive blood pressure reduction in ICH, systolic blood pressure should be controlled at 130–150 mmHg (12). Notably, cerebral venous outflow disorder also has a significant impact on the pathological mechanisms of ICH. Some researchers have found that cerebral venous outflow disorders are closely related to cerebrospinal fluid dynamics (13), especially in the internal jugular vein (IJV), which is the main route of brain drainage (14). Feng et al. (14) found that among patients with ICH, those with positive internal jugular venous reflux had larger perihematomal edema (PHE) volume than those with negative internal jugular venous reflux and demonstrated a positive correlation between jugular venous reflux (JVR) and PHE volume. When JVR worsens, it can further increase ICP, reduce CBF and CPP, aggravate vasogenic edema, and reduce cerebral oxygenation (14, 15). In general, secondary injuries can include vasogenic edema, neuroinflammatory reactions, blood–brain barrier damage, decompensation of cerebral blood flow autoregulation, excessive lowering of blood pressure, and cerebral venous return disorder, which are related. The pathological mechanisms are different, but to a certain extent, they can synergistically aggravate pathological reactions such as brain edema, neuroinflammatory reactions, high ICP, low CPP, oxidative stress, cell apoptosis, and destruction of the blood–brain barrier after ICH. From a comprehensive literature review, we believe that ischemia and hypoxia after ICH intersect and interact with these pathological reactions (16, 17). (The details are shown in Figure 1.)
A green stroke channel has been established clinically, and the slogan “time is brain” has been put forward because ICH is dangerous and progresses rapidly. Although there is currently no specific clinical treatment for ICH, the pathological basis of ICH can be explored to improve patient prognosis. Recently, high-concentration oxygen therapy has become increasingly active in the public eye. This emerging and effective intervention can alleviate ischemic hypoxic conditions after ICH, reduce intracranial pressure and cerebral edema, and improve neuroinflammatory reactions and other adverse effects. This study aimed to explore the progress in the efficacy of high-concentration oxygen therapy for ICH. Clinically, common high-concentration oxygen therapies used after ICH can be categorized into hyperbaric oxygen (HBO) and normobaric oxygen (NBO).
 He Zeng
He Zeng Dakai Zeng
Dakai Zeng Xiaoping Yin
Xiaoping Yin Wumiao Zhang
Wumiao Zhang Moxin Wu
Moxin Wu Zhiying Chen
Zhiying Chen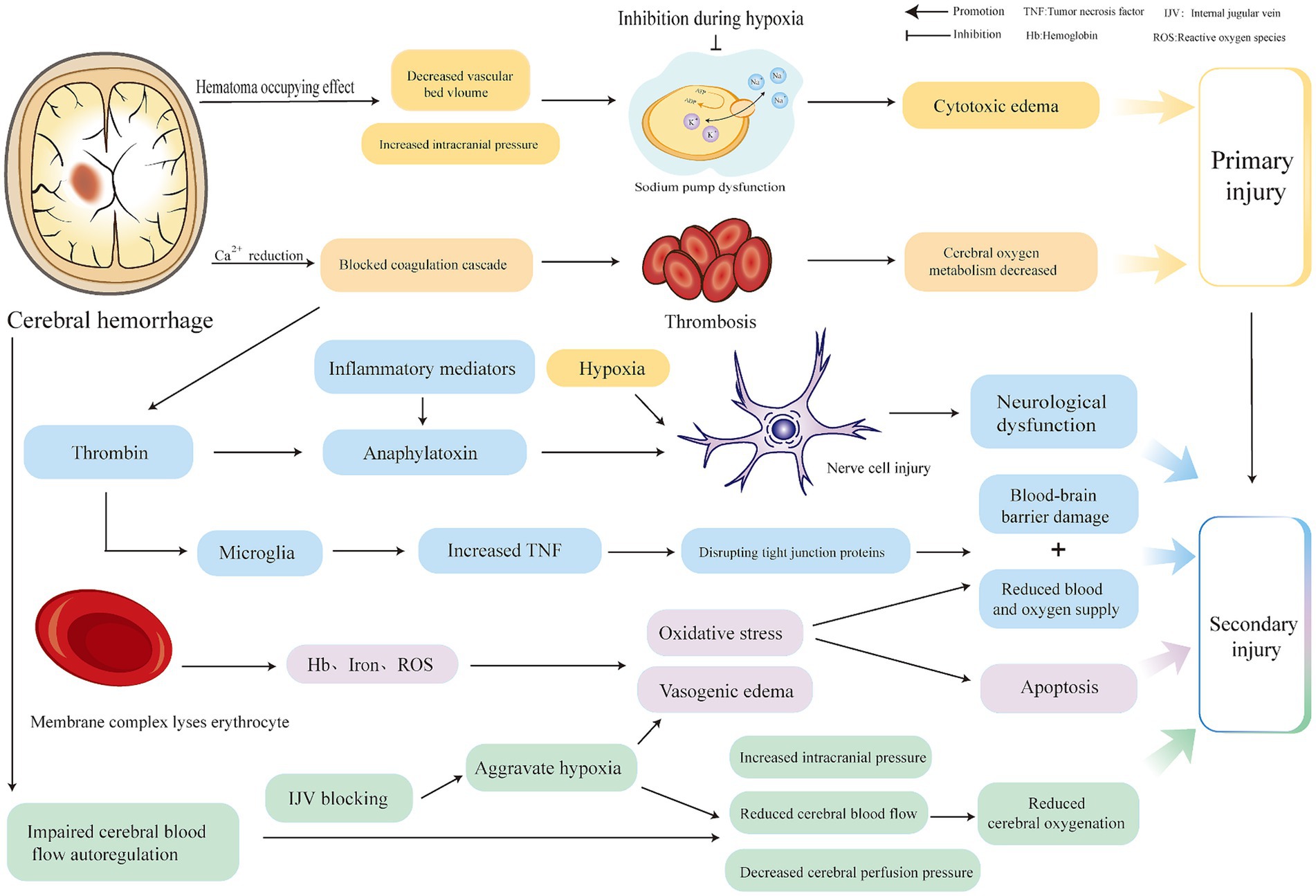
No comments:
Post a Comment