http://www.theguardian.com/science/neurophilosophy/2015/sep/17/neurons-dynamically-switch-identity-in-response-to-brain-activity
A hitherto unknown form of neuroplasticity discovered by researchers at King’s College London helps to resolve a long-standing crisis of neuronal identity
It is often said that the human brain is the most complex object in
the known universe, and for good reason. Even the apparently simple task
of compiling a census of the different types of cells it contains has
proven to be extremely difficult. Researchers still can’t agree on the
best way to classify the numerous sub-types of neurons, and different
methods produce different results, so estimates range from several
hundred to over a thousand.
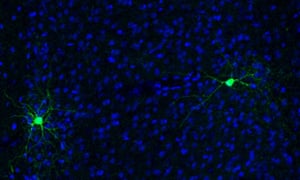
Basket cells illustrate this neuronal identity crisis perfectly. They are currently sub-divided into multiple different types, according to their electrical properties and molecular profiles. After nearly ten years of detective work, researchers at King’s College London now reveal them to be masters of disguise. In a surprising new study, they show that these cells can dynamically switch from one identity to another in response to neuronal network activity.
Basket cells are a type of interneuron, which are found scattered throughout the cerebral cortex, hippocampus, and cerebellum, and make up about 5% of the total number of cells in these brain regions. They form local circuits with each other and with pyramidal neurons, the much larger and more numerous cells that transmit information to distant parts of the brain, and synthesize the inhibitory neurotransmitter GABA, which dampens pyramidal cell activity when released.
These enigmatic cells are thought to exist in more than twenty different types, the best known being the fast-spiking ones, which respond rapidly to incoming signals, and slower ones, which respond after a delay. During brain development, immature forms of all types of basket cells are created in a structure called the medial ganglionic eminence, along with various other types of brain cells. They then migrate into the developing cerebral cortex, before going on to form synaptic connections with other cells.
Back in 2007, Oscar Marín of the MRC Centre for Developmental Neurobiology and his colleagues reported that a protein called Er81 is found in immature medial ganglionic eminence cells, and also at varying levels in small numbers of cells throughout the cortex. Er81 is a master controller that orchestrates the activity of developmental genes. When synthesized by a cell, it enters the nucleus, binds to specific DNA sequences in its target genes and helps young brain cells to find their place and purpose, by switching sets of these genes on and off at different times and places. It is, for example, needed for specifying the identity of sensory and motor neurons, and also controls how they connect with each other in the spinal cord.
Its function in basket cells is unknown, however, and so this new study, led by research associate Nathalie Dehorer, sought to investigate the possibility that Er81 specifies their identity, too. First, they examined slices of tissue from the cortex of genetically engineered mice whose basket cells produce green fluorescent protein. First, they used microelectrodes to record the cells’ electrical activity, confirming that some of the fluroescently-labelled neurons were fast-spiking basket cells, and some of them the slow ones. Another experiment revealed that while Er81 is present at high levels in slow basket cells, it seems to be completely missing from the fast-spiking ones.
Next, the researchers created their own genetically engineered mice, in order to delete the Er81 gene in specific brain regions and at different times of the animals’ lives. Deleting the gene from the medial ganglionic eminence in embryonic mice had no effect on the number of basket cells, or their distribution within the cerebral cortex, indicating that the protein is not needed for their migration or for the earliest stages of specifying their identity. They noticed, however, that most of the basket cells in these animals were fast-spiking ones, suggesting that Er81 is needed to uphold the identity of the slow ones.
To test this, the researchers created another strain of mice and deleted the gene from basket cells in the cortex of adult animals. Examination of the brain tissue revealed that this caused an almost complete loss of slow basket cells, due to changes in the activity of potassium channel genes, which control the cells’ electrical properties, as well as a major rearrangement of the synaptic inputs they receive from other cells.
This time, they found that levels of Er81 within the nucleus were directly related to the length of the delay in basket cell responses, and that neuronal network activity markedly alters the ratio of fast-spiking to slow basket cells. And although the Er81 molecule is missing from the fast cells, all basket cell sub-types contain Er81 transcripts, the copies of the genetic blueprint that are exported from the nucleus to be used for protein synthesis.
Thus, Er81 appears to act as a molecular switch that can alter the electrical properties of basket cells, enabling them to dynamically morph between fast and slow states, in response to changes in neuronal network activity. The findings, published in the journal Science last week, suggest the basket cells exist on a continuum, rather than as discrete sub-types, that they are permanently tuned to neuronal network activity, and that they are continuously adapting to it by flipping between their fast and slow states.
Until recently, neuronal identity in the adult brain was thought to be permanently fixed, but this is not the case. We now know, for example, that mature neurons can swap one neurotransmitter for another, and a study earlier this year showed that deletion of another master controller, called LHX2, respecifies the identity of touch neurons in the mouse cortex so that they process other kinds of information. These new findings provide yet more evidence that the adult brain is far more malleable than previously thought,
Basket cells are thought to make up no more than 5% of the total number of cells in the cerebral cortex, but nevertheless they are believed to be vital for proper neuronal circuit function. Each forms synapses with many tens of thousands of pyramidal neurons, and they form networks that regulate the collective activity of pyramidal cell populations before it is transmitted to other parts of the brain. The findings could, therefore, advance our understanding of how basket cells regulate neuronal network activity, and of neurological conditions such as epilepsy, which may be at least partly due to disrupted interneuron function.
Reference: Dehorter, N., et al. (2015). Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science, 349: 1216-20. [Abstract]
Basket cells illustrate this neuronal identity crisis perfectly. They are currently sub-divided into multiple different types, according to their electrical properties and molecular profiles. After nearly ten years of detective work, researchers at King’s College London now reveal them to be masters of disguise. In a surprising new study, they show that these cells can dynamically switch from one identity to another in response to neuronal network activity.
Basket cells are a type of interneuron, which are found scattered throughout the cerebral cortex, hippocampus, and cerebellum, and make up about 5% of the total number of cells in these brain regions. They form local circuits with each other and with pyramidal neurons, the much larger and more numerous cells that transmit information to distant parts of the brain, and synthesize the inhibitory neurotransmitter GABA, which dampens pyramidal cell activity when released.
These enigmatic cells are thought to exist in more than twenty different types, the best known being the fast-spiking ones, which respond rapidly to incoming signals, and slower ones, which respond after a delay. During brain development, immature forms of all types of basket cells are created in a structure called the medial ganglionic eminence, along with various other types of brain cells. They then migrate into the developing cerebral cortex, before going on to form synaptic connections with other cells.
Back in 2007, Oscar Marín of the MRC Centre for Developmental Neurobiology and his colleagues reported that a protein called Er81 is found in immature medial ganglionic eminence cells, and also at varying levels in small numbers of cells throughout the cortex. Er81 is a master controller that orchestrates the activity of developmental genes. When synthesized by a cell, it enters the nucleus, binds to specific DNA sequences in its target genes and helps young brain cells to find their place and purpose, by switching sets of these genes on and off at different times and places. It is, for example, needed for specifying the identity of sensory and motor neurons, and also controls how they connect with each other in the spinal cord.
Its function in basket cells is unknown, however, and so this new study, led by research associate Nathalie Dehorer, sought to investigate the possibility that Er81 specifies their identity, too. First, they examined slices of tissue from the cortex of genetically engineered mice whose basket cells produce green fluorescent protein. First, they used microelectrodes to record the cells’ electrical activity, confirming that some of the fluroescently-labelled neurons were fast-spiking basket cells, and some of them the slow ones. Another experiment revealed that while Er81 is present at high levels in slow basket cells, it seems to be completely missing from the fast-spiking ones.
Next, the researchers created their own genetically engineered mice, in order to delete the Er81 gene in specific brain regions and at different times of the animals’ lives. Deleting the gene from the medial ganglionic eminence in embryonic mice had no effect on the number of basket cells, or their distribution within the cerebral cortex, indicating that the protein is not needed for their migration or for the earliest stages of specifying their identity. They noticed, however, that most of the basket cells in these animals were fast-spiking ones, suggesting that Er81 is needed to uphold the identity of the slow ones.
To test this, the researchers created another strain of mice and deleted the gene from basket cells in the cortex of adult animals. Examination of the brain tissue revealed that this caused an almost complete loss of slow basket cells, due to changes in the activity of potassium channel genes, which control the cells’ electrical properties, as well as a major rearrangement of the synaptic inputs they receive from other cells.
This time, they found that levels of Er81 within the nucleus were directly related to the length of the delay in basket cell responses, and that neuronal network activity markedly alters the ratio of fast-spiking to slow basket cells. And although the Er81 molecule is missing from the fast cells, all basket cell sub-types contain Er81 transcripts, the copies of the genetic blueprint that are exported from the nucleus to be used for protein synthesis.
Thus, Er81 appears to act as a molecular switch that can alter the electrical properties of basket cells, enabling them to dynamically morph between fast and slow states, in response to changes in neuronal network activity. The findings, published in the journal Science last week, suggest the basket cells exist on a continuum, rather than as discrete sub-types, that they are permanently tuned to neuronal network activity, and that they are continuously adapting to it by flipping between their fast and slow states.
Until recently, neuronal identity in the adult brain was thought to be permanently fixed, but this is not the case. We now know, for example, that mature neurons can swap one neurotransmitter for another, and a study earlier this year showed that deletion of another master controller, called LHX2, respecifies the identity of touch neurons in the mouse cortex so that they process other kinds of information. These new findings provide yet more evidence that the adult brain is far more malleable than previously thought,
Basket cells are thought to make up no more than 5% of the total number of cells in the cerebral cortex, but nevertheless they are believed to be vital for proper neuronal circuit function. Each forms synapses with many tens of thousands of pyramidal neurons, and they form networks that regulate the collective activity of pyramidal cell populations before it is transmitted to other parts of the brain. The findings could, therefore, advance our understanding of how basket cells regulate neuronal network activity, and of neurological conditions such as epilepsy, which may be at least partly due to disrupted interneuron function.
Reference: Dehorter, N., et al. (2015). Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science, 349: 1216-20. [Abstract]
No comments:
Post a Comment