I'm sure stroke survivors would rather have the non-surgery options. Ask your competent? doctor why surgery.
Dorset Embarks on Revolutionary Stroke Recovery Trial Utilizing Earpiece Technology February 2024
Non-invasive VNS approach could enhance post-stroke recovery outcomes August 2023
The latest here:
Stroke changed teacher’s life: How new electrical device is helping her move
December 3, 2024 at 4:00 a.m.

MIAMI -- As her students finished their online exam, Arlet Lara got up to make a cafe con leche.
Her 16-year-old son found her on the kitchen floor. First, he called Dad in a panic. Then 911.
"I had a stroke and my life made a 180-degree turn," Lara told the Miami Herald, recalling the medical scare she experienced in May 2020 in the early months of the COVID pandemic.
"The stroke affected my left side of the body," the North Miami woman and former high school math teacher said.
Lara, an avid runner and gym goer, couldn't even walk.
"It was hard," the 50-year-old mom said.
After years of rehabilitation therapy and a foot surgery, Lara can walk again. But she still struggles with moving.
This summer, she became the first patient in South Florida to get an implant of a new and only FDA-approved nerve stimulation device designed to help ischemic stroke survivors regain movement in their arms and hands.
This first procedure was at Jackson Memorial Hospital in Miami. Lara's rehab was at at the Christine E. Lynn Rehabilitation Center for The Miami Project to Cure Paralysis, part of a partnership between Jackson Health System and UHealth.
Every year, thousands in the United States have a stroke, with one occurring every 40 seconds, according to the U.S. Centers for Disease Control and Prevention. The majority of strokes are ischemic, often caused by blood clots that obstruct blood flow to the brain.
For survivors, most of whom are left with some level of disability, the Vivistim Paired VNS System, the device implanted in Lara's chest, could be a game changer in recovery, said Dr. Robert Starke, a UHealth neurosurgeon and interventional neuroradiologist. He also serves as co-director of endovascular neurosurgery at Jackson Memorial Hospital, part of Miami-Dade's public hospital system.
What to know about the stroke device
The Vivistim Paired VNS System is a small pacemaker-like device implanted in the upper chest and neck area. Patients can go home the same day.
The U.S. Food and Drug Administration approved the stroke rehabilitation system in 2021 to be used alongside post-ischemic stroke rehabilitation therapy to treat moderate to severe mobility issues in hands and arms.
Lara's occupational therapist can activate the device during rehabilitation sessions to electrically stimulate the vagus nerve, which runs from the brain down to the abdomen and regulates various parts of the body's nervous system. The electrical stimulation rewires the brain to improve a stroke survivor's ability to move their arms and hands.
How it worked on the first Jackson patient
Lara also has a magnet she can use to activate the device when she wants to practice at home. Her therapy consists of repetitive tasks, including coloring, pinching cubes and grabbing and releasing cylindrical shapes.
After several weeks of rehabilitation therapy with the device, Lara has seen improvement.
"Little by little, I'm noticing that my hand is getting stronger. I am already able to brush my teeth with the left hand," she told the Miami Herald in September.
Since then, Lara has finished the initial six-week Vivitism therapy program, and is continuing to use the device in her rehabilitation therapy. She continues to improve and can now eat better with her left hand and can brush her hair with less difficulty, according to her occupational therapist, Neil Batungbakal.
Lara learned about the device through an online group for stroke survivors and contacted the company to inquire. She then connected them with her Jackson medical team. Now a year later, the device is available to Jackson patients. So far, four patients have received the implant at Jackson.
Starke sees the device as an opportunity to help bring survivors one step closer to regaining full mobility. Strokes are a leading cause of disability worldwide.
While most stroke survivors can usually recover some function through treatment and rehabilitation, they tend to hit a "major plateau" after the first six months of recovery, he said. Vivistim, when paired with rehabilitation therapy, could change that.
Jackson Health said results of a clinical trial published in the peer-reviewed medical journal The Lancet in 2021 showed that the device, "when paired with high-repetition, task-specific occupational or physical therapy, helps generate two to three times more hand and arm function for stroke survivors than rehabilitation therapy alone."
The device has even shown to benefit patients 20 years from their original stroke, according to Starke.
"So now a lot of these patients that had strokes 10-15 years ago that thought that they would never be able to use their arm in any sort of real functional way are now able to have a real meaningful function, which is pretty tremendous," Starke said.
More about the device
Vivistim's vagus-nerve stimulation technology was developed by researchers at the University of Texas at Dallas' Texas Biomedical Device Center and is being sold commercially by Austin-based MicroTransponder, a company started by university graduates. Similar devices are used to treat epilepsy and depression.
For Lara, the device is a new tool to help her recovery journey.
"Everything becomes a challenge so we are working with small things every day because I want to get back as many functions as possible," Lara said.
Patients interested in Vivistim should speak with their doctor to check their eligibility. The FDA said patients should make sure to discuss any prior medical history, including concurrent forms of brain stimulation, current diathermy treatment, previous brain surgery, depression, respiratory diseases and disorders such as asthma, and cardiac abnormalities.
"Adverse events included but were not limited to dysphonia (difficulty speaking), bruising, falling, general hoarseness, general pain, hoarseness after surgery, low mood, muscle pain, fracture, headache, rash, dizziness, throat irritation, urinary tract infection and fatigue," the FDA said.
MicroTransponder says the device is "covered by Medicare, Medicaid, and private insurance with prior authorization on a case-by-case basis."
To learn more about the device, visit vivistim.com.
 Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, right, runs into her rehabilitation neurology
physician Dr. Gemayaret Alvarez, before her physical therapy
appointment on Monday, Sept. 9, 2024, at Lynn Rehabilitation Center at
Jackson Memorial Hospital. The implant is designed to help stroke
survivors regain function in their arms. (Alie Skowronski/Miami
Herald/TNS)
Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, right, runs into her rehabilitation neurology
physician Dr. Gemayaret Alvarez, before her physical therapy
appointment on Monday, Sept. 9, 2024, at Lynn Rehabilitation Center at
Jackson Memorial Hospital. The implant is designed to help stroke
survivors regain function in their arms. (Alie Skowronski/Miami
Herald/TNS) Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant designed to help stroke survivors regain
function in their arms, goes through exercises while her therapist
activates the device during her physical therapy appointment on Monday,
Sept. 9, 2024, at Lynn Rehabilitation Center at Jackson Memorial
Hospital. The activation works as positive reinforcement to her muscles
when she completes the exercise correctly. (Alie Skowronski/Miami
Herald/TNS)
Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant designed to help stroke survivors regain
function in their arms, goes through exercises while her therapist
activates the device during her physical therapy appointment on Monday,
Sept. 9, 2024, at Lynn Rehabilitation Center at Jackson Memorial
Hospital. The activation works as positive reinforcement to her muscles
when she completes the exercise correctly. (Alie Skowronski/Miami
Herald/TNS) Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, does an exercise while Neil Batungbakal,
rehabilitation therapist, activates the implant with the black trigger
during her physical therapy appointment on Monday, Sept. 9, 2024, at
Lynn Rehabilitation Center at Jackson Memorial Hospital. The implant is
designed to help stroke survivors regain function in their arms. The
activation works as positive reinforcement to her muscles when she
completes the exercise correctly. (Alie Skowronski/Miami Herald/TNS)
Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, does an exercise while Neil Batungbakal,
rehabilitation therapist, activates the implant with the black trigger
during her physical therapy appointment on Monday, Sept. 9, 2024, at
Lynn Rehabilitation Center at Jackson Memorial Hospital. The implant is
designed to help stroke survivors regain function in their arms. The
activation works as positive reinforcement to her muscles when she
completes the exercise correctly. (Alie Skowronski/Miami Herald/TNS)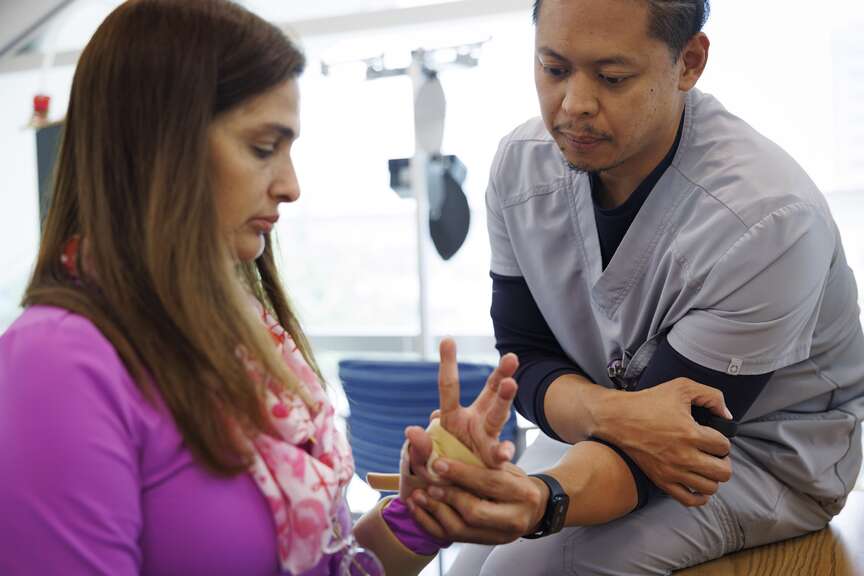 Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, does an exercise while Neil Batungbakal,
rehabilitation therapist, activates the implant with the black trigger
during her physical therapy appointment on Monday, Sept. 9, 2024, at
Lynn Rehabilitation Center at Jackson Memorial Hospital. The activation
works as positive reinforcement to her muscles when she completes the
exercise correctly. (Alie Skowronski/Miami Herald/TNS)
Arlet Lara, the first patient in South Florida to get an FDA-approved
nerve stimulation implant, does an exercise while Neil Batungbakal,
rehabilitation therapist, activates the implant with the black trigger
during her physical therapy appointment on Monday, Sept. 9, 2024, at
Lynn Rehabilitation Center at Jackson Memorial Hospital. The activation
works as positive reinforcement to her muscles when she completes the
exercise correctly. (Alie Skowronski/Miami Herald/TNS)
No comments:
Post a Comment