http://journal.frontiersin.org/article/10.3389/fneur.2015.00121/full?
- 1Neuroscience Section, Department of Neurofarba, University of Florence, Florence, Italy
- 2Stroke Unit, Department of Neurology, Careggi University Hospital, Florence, Italy
- 3Department of Experimental and Clinical Medicine, Atherothrombotic Diseases Center, AOU Careggi, University of Florence, Florence, Italy
- 4Emergency Department Stroke Unit, Department of Neurological Sciences, Sapienza University of Rome, Rome, Italy
- 5SSO Stroke Unit, U.O. Neurologia d.O., DAI di Neuroscienze, Azienda Ospedaliera Integrata, Verona, Italy
- 6Neurology Unit, Valduce General Hospital, Como, Italy
- 7U.O. Neurologia, DAI Neuroscienze-Riabilitazione, Azienda Ospedaliera-Universitaria S. Anna, Ferrara, Italy
- 8U.O. Neurologia, G. Jazzolino Hospital, Vibo Valentia, Italy
- 9Istituto Neurologico Nazionale C. Mondino, Pavia, Italy
- 10U.O.C. Stroke Unit, Dipartimento di Scienze Neurologiche e Neurosensoriali, Azienda Ospedaliera Universitaria Senese, Siena, Italy
- 11Department of Neurosciences, Neurological Clinic, University of Pisa, Pisa, Italy
- 12UOC di Neurologia e “Stroke Unit”, Ospedale San Bortolo, Vicenza, Italy
- 13Neurology Unit, Arcispedale Santa Maria Nuova, Reggio Emilia, Italy
- 14Department of Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON, Canada
- 15Neurology Unit, Misericordia e Dolce Hospital, Prato, Italy
- 16Stroke Unit, Department of Neurology, Ospedale di Circolo e Fondazione Macchi, Varese, Italy
- 17Department of Neurology, Istituti Ospitalieri, Cremona, Italy
- 18Institute of Neuroscience, Italian National Research Council, Florence, Italy
Background: Experimentally, metalloproteinases (MMPs) play a
detrimental role related to the severity of ischemic brain lesions.
Both MMPs activity and function in tissues reflect the balance between
MMPs and tissue inhibitors of metalloproteinases (TIMPs). We aimed to
evaluate the role of MMPs/TIMPs balance in the setting of rtPA-treated
stroke patients.
Methods: Blood was taken before and 24-h after rtPA from
327 patients (mean age 68 years, median NIHSS 11) with acute ischemic
stroke. Delta median values of each MMP/TIMP ratio [(post rtPA
MMP/TIMP-baseline MMP/TIMP)/(baseline MMP/TIMP)] were analyzed related
to symptomatic intracranial hemorrhage (sICH) according to NINDS
criteria, relevant hemorrhagic transformation (HT) defined as confluent
petechiae within the infarcted area or any parenchymal hemorrhage,
stroke subtypes (according to Oxfordshire Community Stroke Project) and
3-month death. The net effect of each MMP/TIMP ratio was estimated by a
logistic regression model including major clinical determinants of
outcomes
Results: Adjusting for major clinical determinants, only
increase in MMP9/TIMP1 and MMP9/TIMP2 ratios remained significantly
associated with sICH (odds ratio [95% confidence interval], 1.67
[1.17–2.38], p = 0.005; 1.74 [1.21–2.49], p = 0.003,
respectively). Only relative increase in MMP9/TIMP1 ratio proved
significantly associated with relevant HT (odds ratio [95% confidence
interval], 1.74 [1.17–2.57], p = 0.006) with a trend toward significance for MMP9/TIMP2 ratio (p = 0.007).
Discussion: Our data add substantial clinical evidence
about the role of MMPs/TIMPs balance in rtPA-treated stroke patients.
These results may serve to generate hypotheses on MMPs inhibitors to be
administered together with rtPA in order to counteract its deleterious
effect.
Introduction
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endopeptidases that are involved in extracellular matrix
(ECM) degradation (1).
The turnover of ECM is regulated by the balance between MMPs and a
group of endogenous proteins called tissue inhibitor of
metalloproteinases (TIMPs) (2).
Active MMPs and some MMP proenzymes form 1:1 complexes with TIMPs and
the unbalance between these two families of molecules appears implicated
in a variety of diseases (3). A list of MMPs and TIMPs with their putative role in acute ischemic stroke is shown in Table S1 in Supplementary Material.
After cerebral ischemia, the general neuronal response
to excitotoxic injury determines the release of pro-inflammatory
cytokines that stimulate the local production of MMPs and TIMPs (4).
In experimental models of brain ischemia, MMPs and MMP/TIMP unbalance
play a detrimental role related to blood–brain barrier (BBB) disruption
leading to hemorrhagic transformation and edema of an ischemic brain
lesion (5).
Circulating levels of MMP9 have been proved associated with poor
outcomes in stroke patients treated with tissue plasminogen activator
(rtPA) (6, 7). Furthermore, recent studies suggest that rtPA adverse effects may be mediated through MMPs upregulation and activation (2).
No clinical study has hitherto considered selectively the effect of the
balance between MMPs and their physiological inhibitor related to
stroke outcomes after thrombolysis. Theoretical effects of rtPA on
MMP/TIMP unbalance have been shown in Figure 1.
FIGURE 1
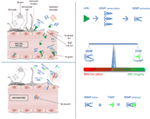 Figure 1. Impact of tissue plasminogen activator on MMP/TIMP unbalance at the neurovascular unit level.
After acute ischemic stroke, rtPA may cross blood–brain barrier (BBB),
enter the brain parenchyma, and thereby damage neurovascular unit
components by promoting metalloproteinase (MMPs) production and
activation. Indeed, unbalance between MMPs and their natural inhibitors
(tissue inhibitors of metalloproteinases, TIMPs) may exacerbate BBB
disruption leading to hemorrhagic transformation and edema of an
ischemic brain lesion.
Figure 1. Impact of tissue plasminogen activator on MMP/TIMP unbalance at the neurovascular unit level.
After acute ischemic stroke, rtPA may cross blood–brain barrier (BBB),
enter the brain parenchyma, and thereby damage neurovascular unit
components by promoting metalloproteinase (MMPs) production and
activation. Indeed, unbalance between MMPs and their natural inhibitors
(tissue inhibitors of metalloproteinases, TIMPs) may exacerbate BBB
disruption leading to hemorrhagic transformation and edema of an
ischemic brain lesion.
The aim of this study was to
evaluate the effect of MMPs/TIMPs ratio on outcomes of ischemic stroke
in the same cohort of the biological markers associated with acute
ischemic stroke (MAGIC) study. Because MMP inhibition is considered a
possible therapeutic target for stroke patients (8),
a clearer understanding of MMP/TIMP interplay, compared with the effect
of MMPs only, would have important implications for acute stroke
therapies.
More at link.
 Benedetta Piccardi
Benedetta Piccardi
No comments:
Post a Comment