In here is one that is non-invasive, no surgery. Why was this research done with all this earlier stuff? Your mentors and senior researchers incompetently didn't know about it?
deep brain stimulation (10 posts to July 2015)
Deep Brain Stimulation Promising in Post-Stroke Recovery
An early-stage clinical trial has shown that deep brain stimulation (DBS) applied to the cerebellum may aid the recovery of upper limb function after stroke.
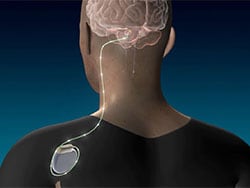
Researchers studied 12 people with moderate to severe upper extremity impairment after stroke who received DBS to the dentate nucleus (DN) of the cerebellum together with rehabilitation and found that 75% of the participants had meaningful response in regaining some function of their paralyzed arms.
PET revealed significant increments in brain metabolism around the part of the brain affected by the stroke.
"Our findings support the safety and feasibility of deep brain stimulation to the cerebellar dentate nucleus as a promising tool for modulation of late-stage neuroplasticity for functional recovery," the authors write.
"The results of the phase I study are promising; but more research is needed. The next step is a phase II clinical trial that is already enrolling patients at Cleveland Clinic," said senior author André Machado, MD, PhD, chairman, Neurological Institute and Charles and Christine Carroll Family Endowed Chair in Functional Neurosurgery, Cleveland Clinic.
The study was published online August 14 in Nature Medicine.
Extending the Neuroplasticity Window
Machado patented the DBS method in stroke recovery, a release from Cleveland Clinic notes. Boston Scientific owns a license to those patents and provided the Vercise DBS systems used in this trial. In 2010, Cleveland Clinic Innovations established Enspire DBS Therapy, Inc, a Cleveland Clinic portfolio company, and is commercializing technology developed at Cleveland Clinic to commercialize the method and it co-funded the study. Machado holds stock options and equity ownership rights with Enspire and serves as the chief scientific officer.
"Stroke is a leading cause of physical disability in the US and around the world; and while physical and occupational therapy work and improve function, about 50% of patients maintain severe levels of disability for life," Machado said.
Neuroplasticity is a "well-documented phenomenon that is associated with gradual spontaneous or therapy-driven improvements in post-stroke motor function," the authors write. But the extent of recovery "varies considerably across individuals" and depends on a variety of factors, the authors write.
"Harnessing the potential of neuroplasticity and modulating its extent and timing remains a major frontier in medicine with vast upside and has been the focus of our group," they continue.
The researchers proposed a new surgical approach for "extending the degree and temporal window of neuroplasticity after ischemic and traumatic insults to the brain."
This approach involves continuous stimulation of the cerebellar DN to "modulate neural activity and ipsilesional cortical excitability through activation of the robust, endogenous dentatothalamocortical pathway."
Machado explained that cerebellar DN "promotes reorganization of the cerebral cortex around the area affected by a stroke and increases the activity levels and metabolism of the cerebral cortex." It also promotes new synapses and the expression of long-term potentiation, a neuroplasticity process.
After over one decade of preclinical studies, the researchers applied this approach to humans for the first time, with the primary goal of determining whether cerebellar DBS, in combination with rehabilitation, is safe and feasible for post-stroke motor deficits.
To do so, they conducted the Electrical Stimulation of the Dentate Nucleus for Upper Extremity Hemiparesis Due to Ischemic Stroke (EDEN) study, focusing on 12 individuals (mean age, 57.4 ± 6.5 years; mean Upper-Extremity Fugl-Meyer Assessment [FM-UE] score, 22.9 ± 6.2 points) with chronic moderate to severe hemiparesis of the upper extremity due to a unilateral middle cerebral artery stroke that took place between 12 and 36 months prior.
Each participant underwent DBS surgery. After discharge and recovery from the surgery, they had 3 months of rehabilitation before the device was turned on and then 4-8 months of rehabilitation combined with DBS. They continued with rehabilitation after discontinuation of stimulation treatment.
Translational Potential
The researchers evaluated changes in motor impairment and function during five key intervals.
- Pre-surgery to post-surgery
- Rehab only (no DBS)
- Experimental DBS plus rehab phase
- 2-month rehab-carryover phase in the absence of DBS
- Long-term follow-up: 10 months after termination of the experimental treatment phase
In addition, they used PET imaging to characterize metabolic changes across ipsilateral perilesional cortex before and after the DBS plus rehab phase, with the DBS turned off.
From pre- to post-surgery, there was a median 0.5-point decrease in FM-UE score, which, although not deemed significant, "supported the safety of the procedure."
There was a "modest" three-point median improvement across the pre-stimulation, rehab-only phase (P = .004). After that, an additional seven-point improvement was observed, when rehabilitation was combined with DN-DBS (P = .0005).
The gain found during the DBS plus rehab phase was most pronounced in participants who entered the study with some distal preservation of motor function compared with those who entered without distal preservation (P = .007).
During the 2-month, rehab-carryover phase (continued rehabilitation as DBS was weaned by weekly 25% amplitude reductions over the first month and then off during the second month), no further change in FM-UE was observed.
The median FM-UE score for the full cohort also remained unchanged at the end of the long-term follow-up phase, "supporting the durability of the previously realized treatment-related gains," the authors report.
Moreover, the "robust functional gains were directly correlated with cortical reorganization evidenced by increased ipsilesional metabolism," they add.
Participants experienced no device failures and no study-related serious adverse events throughout the trial.
"This emerging intervention has shown translational potential to modulate the magnitude of neuroplastic reorganization toward recovery of function and to extend its time window to late phases of disability," they conclude.
Brooks Gross, PhD, program director, National Institute of Neurological Disorders and Stroke (NINDS), agreed. "The safety and feasibility data from this early study, combined with the potential symptom improvements, certainly support the need for additional, larger trials to see if cerebellar DBS is indeed a potential treatment for post-stroke motor impairment," he stated in a news release.
'Exciting Data'
Commenting for Medscape Medical News, Steven Cramer, MD, MSc, Susan and David Wilstein Endowed Chair in Rehabilitation Medicine and professor, Department of Neurology, David Geffen School of Medicine at UCLA, noted that patients with post-stroke disability "have limited options in 2023, in terms of therapies to boost stroke recovery."
This study "provides exciting data on the safety and clinical efficacy of cerebellar deep brain stimulation, along with favorable PET biomarker data." But, as this is a "relatively small study with no control arm, this approach should now proceed to testing in a phase II controlled clinical trial," said Cramer, who is also the medical director of research at the California Rehabilitation Institute in Los Angeles and was not involved with the current study.
This study was supported by the National Institutes of Health Brain Research Through Advancing Innovative Neurotechnologies Initiative and by Enspire DBS, a spin-off company of Cleveland Clinic. Machado serves as chief medical officer and chair of the Scientific Advisory Board for Enspire DBS Therapy and is paid with stock options. As the inventor, A.G.M. will receive portions of commercialization and/or Cleveland Clinic Foundation stock revenue and payments through Cleveland Clinic Foundation with fees deducted. The other authors' disclosures are listed on the original paper. Cramer serves as a consultant for AbbVie, Constant Therapeutics, BrainQ, Myomo, MicroTransponder, Neurolutions, Panaxium, NeuExcell, Elevian, Helius, Omniscient, Brainsgate, Nervgen, Battelle, and TRCare.
Nat Med. Published online August 14, 2023. Abstract
No comments:
Post a Comment