Why not just hire Dr. Watson? The IBM computer already tackling other medical issues. Or don't you know about that?
Dr. Watson for stroke I wrote this in July 2020.
The latest here:
In-Silico Trials for Treatment of Acute Ischemic Stroke
- 1Biomedical Engineering and Physics, Amsterdam University Medical Centers, Amsterdam, Netherlands
- 2Radiology and Nuclear Medicine, Amsterdam University Medical Centers, Amsterdam, Netherlands
- 3Computational Science Lab, Institute for Informatics, Faculty of Science, University of Amsterdam, Amsterdam, Netherlands
Despite improved treatment, a large portion of patients with acute ischemic stroke due to a large vessel occlusion have poor functional outcome. Further research exploring novel treatments and better patient selection has therefore been initiated. The feasibility of new treatments and optimized patient selection are commonly tested in extensive and expensive randomized clinical trials. in-silico trials, computer-based simulation of randomized clinical trials, have been proposed to aid clinical trials. In this white paper, we present our vision and approach to set up in-silico trials focusing on treatment and selection of patients with an acute ischemic stroke. The INSIST project (IN-Silico trials for treatment of acute Ischemic STroke, www.insist-h2020.eu) is a collaboration of multiple experts in computational science, cardiovascular biology, biophysics, biomedical engineering, epidemiology, radiology, and neurology. INSIST will generate virtual populations of acute ischemic stroke patients based on anonymized data from the recent stroke trials and registry, and build on the existing and emerging in-silico models for acute ischemic stroke, its treatment (thrombolysis and thrombectomy) and the resulting perfusion changes. These models will be used to design a platform for in-silico trials that will be validated with existing data and be used to provide a proof of concept of the potential efficacy of this emerging technology. The platform will be used for preliminary evaluation of the potential suitability and safety of medication, new thrombectomy device configurations and methods to select patient subpopulations for better treatment outcome. This could allow generating, exploring and refining relavant hypotheses on potential causal pathways (which may follow from the evidence obtained from clinical trials) and improving clinical trial design. Importantly, the findings of the in-silico trials will require validation under the controlled settings of randomized clinical trials.
Introduction
Endovascular treatment (EVT) has become the standard of care for patients with acute ischemic stroke (AIS) after its benefit was demonstrated by multiple randomized clinical trials (RCTs) (1). Despite improved functional outcome after EVT, up to 66% patients have an unfavorable outcome and remain functionally dependent (1–3). Functional outcome, generally assessed 90 days after stroke onset, predominantly depends on the patient's baseline characteristics including but not limiting to age (4), previous comorbidities (5), stroke severity (4, 6), collateral capacity (7, 8), and thrombus characteristics (9, 10). Delay to receive care strongly reduces the effect of treatment (11–13). Furthermore, ischemic lesion characteristics like volume and location, before and after treatment are also known to be strong predictors of functional outcome after 90 days (14–19).
New AIS trials are focusing on testing new thrombolytics, improved stent designs and testing the applicability of thrombectomy to previously understudied patient sub-groups. However, not more than 10% of the compounds that are tested in clinical trials get launched in the market (20). By design, RCTs do not serve the purpose of explaining the ineffectiveness of treatments. However, this is a task that could be performed with in-silico approaches (21). Further analysis to explain the established efficacy of a treatment by in-silico methods may allow for generation of potential hypotheses. Before these can be introduced in clinical practice, valiation by RCTs is mandatory. Computational or in-silico modeling is playing an increasing role in research and development of biomedical products and is acknowledged as an alternative to animal studies in some preclinical trials by regulators (21–23). Statistical models that accurately describe the most important patient characteristics can generate “virtual patients.” Combining such virtual patient populations with in-silico models (ISMs) of disease and treatment will help to set up in-silico trials (ISTs). In such ISTs, virtual patients receive virtual treatments and effect of treatment on clinical outcome is estimated (21). This project aims to develop a platform that enables the execution of ISTs for AIS. The proposed IST platform aims to be a proof-of-concept to investigate the extent to which in-silico modeling can accurately simulate bench-testing, animal testing, and clinical trial results. After validating the proof-of-concept, some plausible hypotheses may emerge due to the hypotheses-generating nature of this approach. Although ISTs will not allow for testing these hypotheses, they will be useful in optimizing trial design, may provide potential explanation into the causes of (un-) planned effects including less probable clinical situations (21). We envision that developing such a platform can considerably contribute towards a depper understanding of the etiology and pathophysiology of AIS and its treatment effects at the patient and population levels. In the following sections, we describe a quantitative approach to develop a platform that can execute, validate ISTs for AIS, generate and refine hypotheses on the potential successfulness of new treatments, the suitability of treatments for specific patient populations and to provide tools for in-silico evaluation of trial design modeling.
Methods
To develop and validate a platform to execute an IST, we intend to implement a 3 fold approach. We want to generate virtual populations of AIS patients and develop ISMs for (1) thrombosis and thrombolysis, (2) intra-arterial thrombectomy and (3) microvascular perfusion, cell death, and recovery of brain tissue after reperfusion based on anonymized clinical, imaging, and thrombus histopathological data from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial, the MR CLEAN Registry and the HERMES collaboration (1, 2, 24). We will validate these ISMs using laboratory experiments and available anonymized clinical data. We aim to apply these ISMs to virtual patient populations with AIS with the goal to generate an IST platform, followed by validation and application of the IST platform.
Patient Population
Anonymized baseline (clinical and imaging) data, treatment characteristics and outcome (clinical and imaging) data from patients included in the MR CLEAN trial (2). MR CLEAN Registry (24) and the HERMES collaboration (1), totaling over 4,500 patients will be used to develop, execute, and validate the ISMs and ISTs. Anonymized data from on-going RCTs in AIS patients within the Collaboration for New Treatments of Acute Ischemic Stroke (CONTRAST) consortium (www.contrast-consortium.nl) comprising of ~2,500 patients will also be included in this project. The anonymized data from the HERMES (1) and CONTRAST collaboration will be used to validate the ISTs.
Design
The IST consists of four main software modules (Figure 1). The first module generates virtual populations of AIS patients; the second simulates treatment and brain tissue injury; the third estimates outcome for each individual virtual AIS patient and the final module assembles all results and reports the outcome.
 Praneeta R. Konduri
Praneeta R. Konduri Henk A. Marquering
Henk A. Marquering Ed E. van Bavel
Ed E. van Bavel Alfons Hoekstra
Alfons Hoekstra Charles B. L. M. Majoie
Charles B. L. M. Majoie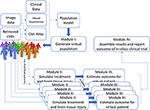
No comments:
Post a Comment