Just maybe you want your doctor to have translated this to hemorrhage stroke patients prior to you getting to the hospital with your hemorrhage. Or do you prefer guesswork?
Escalate and De-Escalate Therapies for Intracranial Pressure Control in Traumatic Brain Injury
- 1Department of Anesthesia and Intensive Care, Ospedale Policlinico San Martino, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) for Oncology and Neuroscience, Genoa, Italy
- 2Department of Neurosurgery, Ospedale Policlinico San Martino, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) for Oncology and Neuroscience, Genoa, Italy
- 3Laboratory of Pulmonary Investigation, Carlos Chagas Filho Biophysics Institute, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 4Rio de Janeiro Network on Neuroinflammation, Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ), Rio de Janeiro, Brazil
- 5Rio de Janeiro Innovation Network in Nanosystems for Health—Nano SAÚDE/Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ), Rio de Janeiro, Brazil
- 6Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health (DINOGMI), University of Genoa, Genoa, Italy
- 7Department of Surgical Sciences and Integral Diagnostics (DISC), University of Genoa, Genoa, Italy
Severe traumatic brain injury (TBI) is frequently associated with an elevation of intracranial pressure (ICP), followed by cerebral perfusion pressure (CPP) reduction. Invasive monitoring of ICP is recommended to guide a step-by-step “staircase approach” which aims to normalize ICP values and reduce the risks of secondary damage. However, if such monitoring is not available clinical examination and radiological criteria should be used. A major concern is how to taper the therapies employed for ICP control. The aim of this manuscript is to review the criteria for escalating and withdrawing therapies in TBI patients. Each step of the staircase approach carries a risk of adverse effects related to the duration of treatment. Tapering of barbiturates should start once ICP control has been achieved for at least 24 h, although a period of 2–12 days is often required. Administration of hyperosmolar fluids should be avoided if ICP is normal. Sedation should be reduced after at least 24 h of controlled ICP to allow neurological examination. Removal of invasive ICP monitoring is suggested after 72 h of normal ICP. For patients who have undergone surgical decompression, cranioplasty represents the final step, and an earlier cranioplasty (15–90 days after decompression) seems to reduce the rate of infection, seizures, and hydrocephalus.
Introduction
Traumatic brain injury (TBI) is a major public health problem, affecting ~64–74 million people and causing 5 million deaths every year, although its true impact seems to be underestimated owing to incomplete data from developing countries (1). TBI carries high rates of hospitalization, morbidity, and mortality. Its pathophysiology is characterized by an elevation of intracranial pressure (ICP), followed by a reduction in cerebral perfusion pressure (CPP) with possible secondary brain damage (2, 3). Monitoring of ICP and surveillance of risk factors for secondary brain injury is recommended by international guidelines (2–4), despite a randomized multicenter international trial investigating monitored and non-monitored patients did not reveal substantial differences in term of outcome (5). Besides, 23–89% of patients are managed without ICP monitoring both for limited resources and expertise, although this can occur also in high-resource countries (6, 7).
A step-by-step approach to treatment escalation, known as the “staircase approach” (3), aiming to obtain normal ICP values and adequate CPP as well as to reduce the risks of secondary damage is recommended for ICP management in patients who present an invasive ICP (inv-ICP) monitoring device (3, 4). Otherwise, in case of non-availability of ICP monitoring, the SIBICC Consensus Protocol for escalating treatments should be followed (6). Hence, two different approaches have been described to manage severe TBI patients, depending on the standard of care, resources-limit, and expertise: (1) pursuing the indications of inv-ICP monitoring, or (2) following brain imaging and clinical examination to escalate therapies. Even though inv-ICP monitoring is not easy to manage, it is recommended by most guidelines (3, 4, 6, 8, 9). Concerning inv-ICP placement, the Brain Trauma Foundation (BTF) and the 2019 SIBICC Consensus Conference leave the decision to the clinician, because previous recommendations were not as strong as needed—previous indications included patients with pathological findings on computed tomography (CT) and a Glasgow Coma Score (GCS) < 8, or impossibility to perform the neurological examination, and patients with normal CT-scan with unavailable neurological examination and two or more of the following risk factors: age > 40 years, hypotension, and abnormal flexion/extension in response to pain (4, 10). TBI is frequently complicated by HICP, which is defined as an increase in ICP over 20–22 mmHg (in inv-ICP monitored patients) (3, 4), while in non-invasively monitored patients who are managed according to imaging and clinical criteria, HICP can be suspected when one major or two minor criteria are met. Major criteria include compressed cisterns (CT classification of Marshall diffuse injury III), midline shift of more than 5 mm (CT classification of Marshall diffuse injury IV), and non-evacuated mass; minor criteria include GCS motor score ≤ 4, pupillary asymmetry, altered pupillary reactivity, midline shift 0–5 mm, and/or lesion of 25 or less cm3 (CT classification of Marshall diffuse injury II). The risk of not monitoring ICP could be an overtreatment of patients with acceptable ICP and an undertreatment of patients with potentially harmful HICP (6, 11). Refractory HICP is defined as intracranial pressure that exceeds 22–25 mmHg for 30 min, or 30 mmHg for 15 min, or 40 mmHg for 1 min (12), and this is the recommended ICP threshold to pursue more aggressive therapies (3, 4). According to the most recent guidelines for the management of TBI, the treatment of HICP is divided into several steps, until the most aggressive including surgical decompression (4, 9, 10). A main concern in neurointensive care unit practice remains how to manage and de-escalate the employed therapies once ICP and CPP targets have been achieved. In fact, each step of treatment escalation carries potential side effects (e.g., hypotension, infection, pneumonia, brain ischemia, electrolyte, and fluid disturbances), frequently related to the duration of treatment (3). Although the management of intracranial hypertension has been widely explored in literature, little evidence is available for withdrawing these treatments and returning to baseline condition.
Therefore, the aim of our narrative review is to briefly describe current practice for the management of intracranial hypertension and to analyze how and when it is recommended to de-escalate HICP therapies in patients with severe TBI, with or without inv-ICP monitoring.
ICP Pathophysiology
The normal ICP value in adults is around 15 mmHg, increasing physiologically during cough or sneeze. The skull is a closed and rigid container, whose volume consists of three components: cerebrospinal fluid, blood, and brain parenchyma. Cranial volumes and pressures are normally self-equilibrated and self-regulated, thereby keeping cerebral blood flow (CBF) constant in case of variation in any one of these compartments or additional volume. Under normal conditions, the compliance curve that describes the relationship between ICP and intracranial volume is exponential. In the first part of the curve, ICP increases slowly, then rises steeply when the compensatory systems are saturated (as in the case of CSF displacement through the foramen magnum, compression of the cerebral venous system, displacement of brain tissue, and herniation syndromes) (3, 8, 9). After TBI, these mechanisms occur in case of an ICP increase and progressive neurological deterioration. ICP values over 20 mmHg (2, 3, 13) or 22 mmHg (4) are considered pathological in adults, and should follow a conservative “staircase approach” or the surgical evacuation of any hematoma if present (3), with the goal of achieving CPP values between 60 and 70 mmHg (4). Any rise in ICP leads to CPP reduction; indeed, CPP is calculated as the mean arterial pressure minus ICP. CBF impairment may progress until the onset of inadequate oxygenation and ischemia (secondary brain injury), which can lead to cytotoxic edema, resulting in further increase in ICP (2–4, 9, 10). Brain trauma or metabolic impairment can cause tissue ischemia, leading to failure of the sodium-potassium pump with subsequent water influx into the cells, followed by brain swelling and lysis. Other compensatory mechanisms are activated after TBI, such as the sympathetic nervous system, which increases cardiac output and blood pressure and triggers systemic vasoconstriction (14). An overview of ICP pathophysiology is depicted in Figure 1.
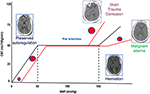
Figure 1. Cerebral autoregulation. Cerebral autoregulation in healthy people is reached at a MAP of 50–150 mmHg and ICP below 20–22 mmHg. After TBI, autoregulation is initially preserved, and compensatory mechanisms act to control ICP and to perfuse the brain (CT scan on the left). When these mechanisms are saturated, cerebral autoregulation is lost, ICP increases, and CBF reduces; if left untreated, this culminates in the worst-case scenario of cerebral herniation (CT scan on the right side). When autoregulation is preserved, pial arterioles dilate in response to ICP increase in order to maintain adequate CBF. When autoregulation is lost, arterioles constrict or dilate causing further reduction of CBF (ischemia) or unnecessary increase of perfusion (hyperemia and contusion evolution or malignant edema). MAP, mean arterial pressure; ICP, intracranial pressure; TBI, traumatic brain injury; CT, computed tomography; CBF, cerebral blood flow, DAD, diffuse axonal damage.
 Denise Battaglini
Denise Battaglini Pasquale Anania
Pasquale Anania Patricia R. M. Rocco
Patricia R. M. Rocco Iole Brunetti1,
Iole Brunetti1,  Gianluigi Zona
Gianluigi Zona Pietro Fiaschi
Pietro Fiaschi
No comments:
Post a Comment