Since you are a knowledge user of stroke you should immediately be contacted by dozens of stroke researchers to participate. Your doctors better have those dozens of names ready to roll off their tongues so you contact them directly and get them setup with Integrated Knowledge Translation. If researcher names are not available then the board of directors is incompetent and has completely the wrong goals for the stroke unit and needs to be fired.
Fit for purpose. Co-production of complex behavioural interventions. A practical guide and exemplar of co-producing a telehealth-delivered exercise intervention for people with stroke
Health Research Policy and Systems volume 20, Article number: 2 (2022)
Abstract
Background
Careful development of interventions using principles of co-production is now recognized as an important step for clinical trial development, but practical guidance on how to do this in practice is lacking. This paper aims (1) provide practical guidance for researchers to co-produce interventions ready for clinical trial by describing the 4-stage process we followed, the challenges experienced and practical tips for researchers wanting to co-produce an intervention for a clinical trial; (2) describe, as an exemplar, the development of our intervention package.
Method
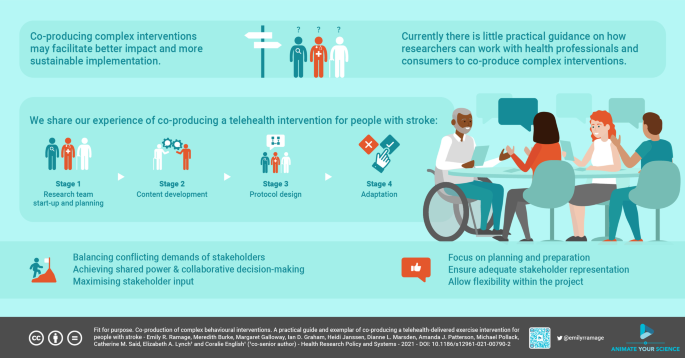
We used an Integrated Knowledge Translation (IKT) approach to co-produce a telehealth-delivered exercise program for people with stroke. The 4-stage process comprised of (1) a start-up planning phase with the co-production team. (2) Content development with knowledge user informants. (3) Design of an intervention protocol. (4) Protocol refinement.
Results and reflections
The four stages of intervention development involved an 11-member co-production team and 32 knowledge user informants. Challenges faced included balancing conflicting demands of different knowledge user informant groups, achieving shared power and collaborative decision making, and optimising knowledge user input. Components incorporated into the telehealth-delivered exercise program through working with knowledge user informants included: increased training for intervention therapists; increased options to tailor the intervention to participant’s needs and preferences; and re-naming of the program. Key practical tips include ways to minimise the power differential between researchers and consumers, and ensure adequate preparation of the co-production team.
Conclusion
Careful planning and a structured process can facilitate co-production of complex interventions ready for clinical trial.
Graphical Abstract
Introduction
Using co-production when developing clinical interventions allows for the perspectives and expertise of different stakeholder groups to be incorporated, thereby enhancing an intervention’s real-world application [1]. Successful healthcare interventions need to be both efficacious and implementable in clinical practice [2]. In the field of stroke recovery and rehabilitation, interventions tend to be complex because they have multiple components and pathway complexity [3]. Poor intervention design and lack of appropriate stakeholder engagement in stroke rehabilitation [4] may be contributing factors to the numerous neutral Phase III clinical trials [5]. Therefore, the Stroke Recovery and Rehabilitation Roundtable expert group have called for improvement in how interventions are developed for clinical trials [5, 6]. Co-production of interventions is important to optimise their success in clinical trial.
There is a lack of consensus of the definition of co-production, for the purpose of this paper we adopt the definition that co-production in research is a “collaborative model of research that includes stakeholders in the research process” [7]. Collaborative research approaches share a number of similarities, one of which is true partnership, i.e. a deep relationship between the researchers and knowledge users throughout the research process to ensure the research benefits all parties [8]. Integrated knowledge translation (IKT) is one co-production research approach in which ‘knowledge users’ are included in the research team in shared partnership [8,9,10]. Knowledge users are people who will administer, influence, or use the research [9, 11]. Knowledge users can include healthcare workers, policymakers who implement research findings, and people with lived experience of the health condition of interest. IKT has previously been used in research involving policymaking, health promotion, knowledge dissemination, evidence-informed decision making, and the development of implementation or quality improvement interventions [12,13,14,15,16]. However, we could not find any published examples that provide practical guidance regarding the use of an IKT co-production approach to design interventions ready for clinical trial.
The aims of this paper are to:
-
1.
Provide practical guidance for researchers to co-produce interventions ready for clinical trial by describing the 4-stage process we followed, the challenges experienced and practical tips for researchers wanting to co-produce an intervention for a clinical trial;
-
2.
Describe, as an exemplar, the development of our intervention package.
The project, its context, contributors and supporting evidence base
Stroke is a leading cause of death and disability worldwide, and most strokes are due to modifiable risk factors (e.g., physical activity, diet, smoking, medication adherence) [17]. Over a quarter of all preventable strokes are secondary strokes [18]. While physical activity is recommended in international guidelines to address secondary stroke risk [19,20,21] the most effective way to deliver physical activity interventions remains unclear. Maintaining adequate physical activity levels after stroke is challenging, and the majority of stroke survivors do not meet minimum activity targets [22, 23]. A physical activity intervention based on what we know physiologically should reduce stroke risk, that is feasible to deliver, and that is also usable and accessible for stroke survivors is needed.
Our paper describes the IKT co-production of a physical activity intervention as part of the development of the Secondary Prevention of Stroke: Study Protocol for a Telehealth-Delivered Physical Activity and Diet Pilot Randomized Trial (ENAbLE-Pilot) [24]. This intervention is currently being tested as part of the ENAbLE pilot trial (trial registration ACTRN12620000189921). An IKT approach to co-production was chosen because of its focus on increasing knowledge use and impact [8], its ability to be applied pragmatically and with the understanding that IKT shares many similarities with other collaborative research approaches [8].
More at link.
No comments:
Post a Comment