I'm sure your competent? doctor knows all this already and has EXACT PROTOCOLS FOR YOUR RECOVERY! NO? So, you DON'T have a functioning stroke doctor, do you? You're not 100% recovered! Why is your doctor getting paid at all? This is all just beating around the bush because NO PROTOCOL FOR GAIT RECOVERY EXISTS!
And that is directly the result of our fucking failures of stroke associations that aren't solving stroke!
Multimodal closed-loop strategies for gait recovery after spinal cord injury and stroke via the integration of robotics and neuromodulation
- 1CHUV, Department of Clinical Neurosciences, University Hospital Lausanne, Lausanne, Switzerland
- 2Bertarelli Foundation Chair in Translational Neural Engineering, Neuro-X Institute, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland
- 3Modular Implantable Neurotechnologies (MINE) Laboratory, Università Vita Salute San Raffaele & Scuola Superiore Sant'Anna, Milan, Italy
Restoring the ability to walk is a priority for individuals with neurological disorders or neurotraumatic injuries, given its significant impact on independence and quality of life. Multimodal closed-loop strategies that integrate robotic assistance and neuromodulation present promising avenues for personalized and physiological gait recovery. These approaches capitalize on residual motor activity, fostering neuroplasticity and motor relearning. This narrative review emphasizes the importance of mobile brain/body imaging (MoBI) for guiding the development of closed-loop systems that integrate volitional brain signals with residual motor activity in stroke and spinal cord injury patients. We explore the potential of rehabilitative and assistive interventional strategies based on robotic devices, such as exoskeletons and powered orthoses, and neuromodulation techniques like functional electrical stimulation and spinal cord stimulation. We highlight the limitations of the single interventional strategies and the potential of the synergistic combination of MoBI, robotics, and neuromodulation for gait recovery. By leveraging residual motor functions and integrating multimodal data from the different domains involved in motor recovery (i.e., brain, muscle, and biomechanics), the complementarity of these interventional strategies has the potential to enable dynamic patient-specific interventions. We outline a perspective framework on how future directions can exploit such integration to promote physiological recovery of lower limb functions and personalized therapies that are both challenging and feasible. Advancing along this path holds the promise of enhancing rehabilitative strategies, ultimately promoting functional recovery and long-term independence for individuals with neuromotor disorders.
1 Introduction
Neurological disorders and neurotraumatic injuries often result in severe motor impairments that significantly impact patients' independence (Oczkowski and Barreca, 1993; Catz et al., 1997; Scivoletto et al., 2013) and quality of life (King, 1996; Dijkers, 1997; Westgren and Levi, 1998). However, residual motor activity, which is preserved in many affected patients, can be harnessed to promote neuroplasticity and enhance muscle strength, ultimately resulting in significant functional improvements (Dobkin, 2004; van Hedel and Dietz, 2010; Langhorne et al., 2011; Nas et al., 2015; Stinear et al., 2020; Somers and Bender-Burnett, 2024). For instance, spinal cord injuries (SCI) are typically subdivided into complete and incomplete, with incomplete SCIs sparing at least some sensorimotor functions (Kang et al., 2018). Even in the case of complete SCIs, where no residual sensorimotor function is observable, some studies have suggested that electrical stimulation and intensive rehabilitation may lead to the restoration of voluntary movements (Angeli et al., 2014). Thus, the absence of observable residual function does not necessarily correspond to a complete lack of neural traffic through the cortico-spinal tract, which may be leveraged through rehabilitation (Wahlgren et al., 2021). Similarly, even though stroke typically causes limb paresis contralateral to the produced brain lesion, substantial functional recovery can be attained by exploiting residual motor functions and the plasticity of nearby brain regions (Virani et al., 2020).
When SCI or stroke results in lower limb paralysis, restoring the ability to walk safely and independently becomes a primary goal for affected individuals. In particular, individuals with SCI-related paraplegia consistently rank gait restoration among their highest priorities, second only to the recovery of bladder, bowel, and sexual functions (Simpson et al., 2012). Although stroke more commonly causes unilateral motor impairments, it is estimated that approximately one-third of stroke survivors do not regain independent ambulation (Hendricks et al., 2002). Among those who do, many continue to exhibit pathological gait asymmetries and reduced walking speed (Veerbeek et al., 2014). Furthermore, even in patients who retain some degree of mobility, residual muscle weakness can contribute to balance deficits, a problem exacerbated by advanced age (Beyaert et al., 2015). While regaining the ability to walk is a central aim of rehabilitation in individuals with lower limb paralysis, even achieving upright standing can yield systemic benefits. These include improvements in cardiovascular regulation (Dunn et al., 1998; Eng et al., 2001; Edwards and Layne, 2007), as well as enhanced bowel (Dunn et al., 1998; Walter et al., 1999; Eng et al., 2001; Hoenig et al., 2001; Netz et al., 2007) and urinary function (Dunn et al., 1998; Walter et al., 1999; Eng et al., 2001).
In recent years, a variety of technology-based interventional strategies have been explored as add-ons to traditional physical therapy for the rehabilitation of lower limb function. In this context, here we focus on two key approaches: powered orthoses and exoskeletons, robotic devices designed to provide mechanical support and passive movement to paralyzed limbs (Herr, 2009), and neuromodulation, which facilitates muscle contractions through the electrical stimulation of the neuromuscular system (Hamid and Hayek, 2008; Popović et al., 2009). Initial proof-of-concept studies have demonstrated the potential of these technologies to restore gait (Asselin et al., 2016; Wagner et al., 2018; Haufe et al., 2020; Romeni et al., 2025) and to support the execution of basic functional tasks such as standing (Hankov et al., 2025), sit-to-stand transitions (Li et al., 2023; Romeni et al., 2025), and stair climbing (Hankov et al., 2025; Romeni et al., 2025). These encouraging results have catalyzed efforts to translate such technologies into real-world applications and activities of daily living (van Dijsseldonk et al., 2020; Rowald et al., 2022), which require more sophisticated control strategies as well as improvements in portability and ease of use in unstructured environments.
Over time, various control strategies have been developed with the dual aim of enabling intuitive and continuous user-driven control to enhance device usability and acceptance (Semprini et al., 2022) and promoting activity-dependent plasticity in the nervous system to maximize neurological recovery (Roy et al., 2012; Mrachacz-Kersting et al., 2019). Wearable mobile brain/body imaging (MoBI) systems, capable of continuously capturing high-density brain and muscle signals along with body movement's kinematics, offer a comprehensive way to optimize and adapt the control of interventional devices (He et al., 2018b) (Figure 1).
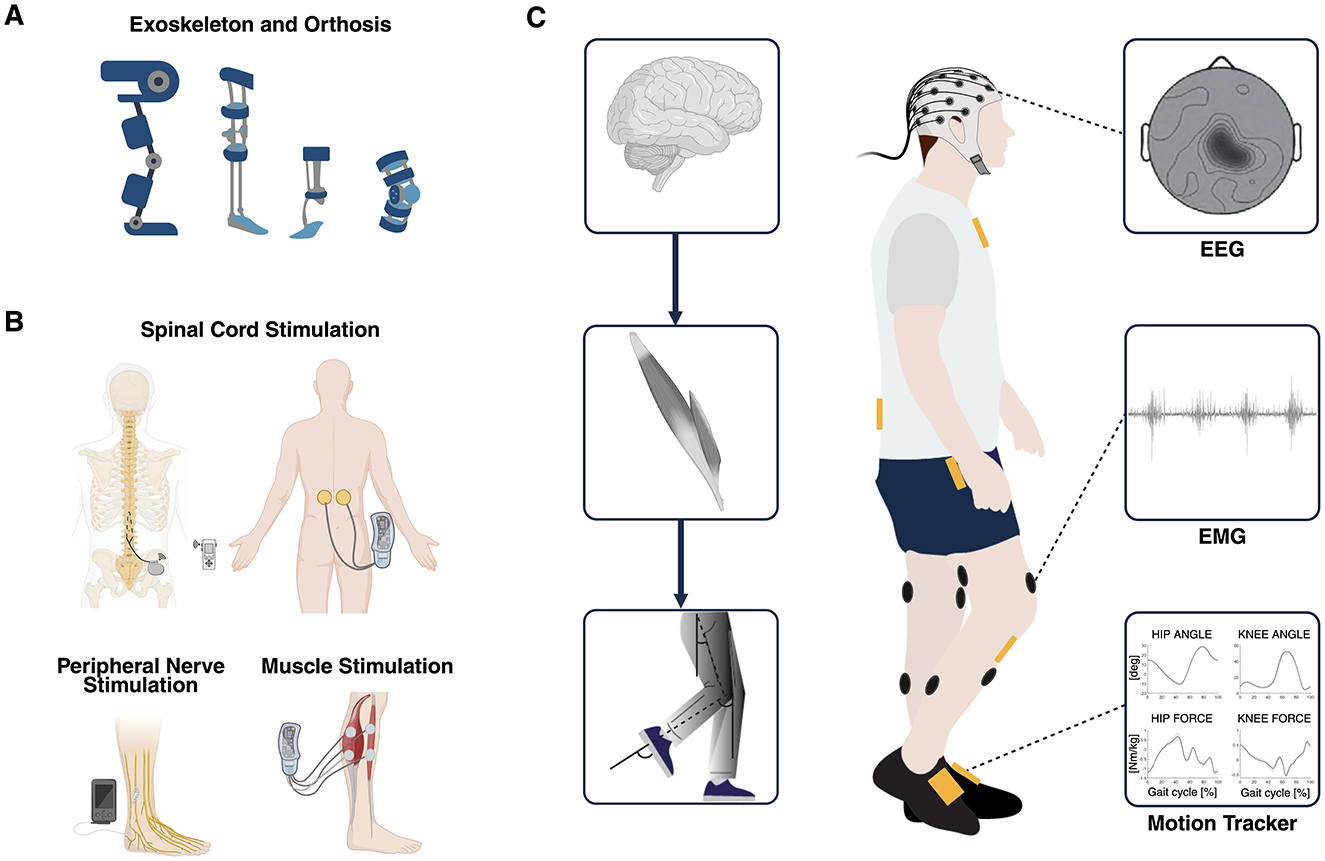
Figure 1. (A) Overview of robot-assisted devices such as exoskeletons and powered orthoses used as established interventional strategies for gait recovery. (B) Overview of electrical stimulation of the spinal cord, the nerves, and the muscles used as established interventional strategies for gait recovery. (C) Multimodal data used to simultaneously explore different domains of the hierarchical organization of the neuromusculoskeletal system: brain, muscles, and biomechanics (MoBI framework). Multimodal biomarkers of residual motor activity can be extracted from EEG, EMG, and kinematics signals and can be exploited to control in real-time different closed-loop interventions aimed at recovering walking through personalized assistance and therapy. MoBI, mobile brain/body imaging; EEG, electroencephalography; EMG, electromyography. Created with BioRender. de Seta V and Romeni S (2025). https://BioRender.com.
In this narrative review, we aim to explore how robotic devices and neuromodulation can be controlled for assistive and rehabilitative interventions, and how different interventional strategies can be integrated to provide personalized gait rehabilitation, leveraging complementary mechanisms of action. First, we will present the state-of-the-art in robotic devices and neuromodulation for lower limb movement restoration; then, we will describe how patients' residual motor activity recorded through various approaches exploiting kinematic, muscle, and neural signals has been used in the past to control such technologies. Finally, we will provide indications and highlight potential issues in the integration of MoBI techniques with different combinations of robotic devices and neuromodulation technologies to achieve functional and physiological recovery of lower limb abilities. The main goal of this review is to provide an overview of rehabilitative interventions for the recovery of lower limb motor functions through the exploitation of residual motor functions, robotic devices, neuromodulation, and monitoring of neurophysiological correlates of movement.
More at link.
 Valeria de Seta
Valeria de Seta Simone Romeni
Simone Romeni
No comments:
Post a Comment