By using pleiotropic as an excuse they completely failed at producing anything of use for survivors. It will require more followup research which will never occur.
Myricetin as a Promising Molecule for the Treatment of Post-Ischemic Brain Neurodegeneration
1
Laboratory of Ischemic and
Neurodegenerative Brain Research, Mossakowski Medical Research
Institute, Polish Academy of Sciences, 02-106 Warsaw, Poland
2
Department of Pathophysiology, Medical University of Lublin, 20-090 Lublin, Poland
*
Author to whom correspondence should be addressed.
Academic Editor: Maria Antonietta Panaro
Nutrients 2021, 13(2), 342; https://doi.org/10.3390/nu13020342
Received: 15 December 2020 / Revised: 17 January 2021 / Accepted: 20 January 2021 / Published: 24 January 2021
(This article belongs to the Special Issue Bioactive Natural Compounds for Therapeutic and Nutraceutical Applications in Neurodegeneration
)
The available drug therapy for post-ischemic neurodegeneration of the
brain is symptomatic. This review provides an evaluation of possible
dietary therapy for post-ischemic neurodegeneration with myricetin. The
purpose of this review was to provide a comprehensive overview of what
scientists have done regarding the benefits of myricetin in
post-ischemic neurodegeneration. The data in this article contribute to a
better understanding of the potential benefits of myricetin in the
treatment of post-ischemic brain neurodegeneration, and inform
physicians, scientists and patients, as well as their caregivers, about
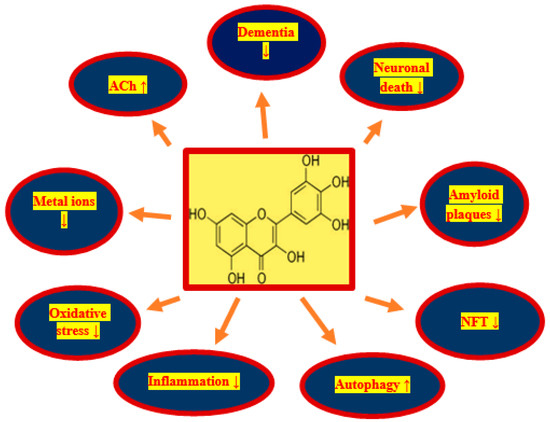
treatment options. Due to the pleiotropic properties of myricetin,
including anti-amyloid, anti-phosphorylation of tau protein,
anti-inflammatory, anti-oxidant and autophagous, as well as increasing
acetylcholine, myricetin is a promising candidate for treatment after
ischemia brain neurodegeneration with full-blown dementia. In this way,
it may gain interest as a potential substance for the prophylaxis of the
development of post-ischemic brain neurodegeneration. It is a safe
substance, commercially available, inexpensive and registered as a
pro-health product in the US and Europe. Taken together, the evidence
available in the review on the therapeutic potential of myricetin
provides helpful insight into the potential clinical utility of
myricetin in treating neurodegenerative disorders with full-blown
dementia. Therefore, myricetin may be a promising complementary agent in
the future against the development of post-ischemic brain
neurodegeneration. Indeed, there is a scientific rationale for the use
of myricetin in the prevention and treatment of brain neurodegeneration
caused by ischemia.
View Full-Text
Keywords:
brain ischemia; myricetin; amyloid; tau protein; autophagy; metal ion; oxidative stress; neuroinflammation; acetylcholine; neurodegeneration; dementia; therapy
▼
Show Figures
This is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited


No comments:
Post a Comment