With new blood vessel formation needed to recover from your stroke, is your incompetent doctor not ready with protocols to accomplish that?
Do you prefer your doctor incompetence NOT KNOWING? OR NOT DOING? Because your doctor is incompetent if nothing has been done, in my opinion.
Targeting vascular endothelial growth receptor-2 (VEGFR-2): structural biology, functional insights, and therapeutic resistance
Abstract
Angiogenesis, the process of new blood vessel formation, is a fundamental physiological process implicated in several pathological disorders. The vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are crucial for angiogenesis and vasculogenesis. Among them, the tyrosine kinase receptor VEGFR-2 is primarily expressed in endothelial cells (ECs). These cells regulate various physiological responses, including differentiation, cell proliferation, migration, and survival, by binding to VEGF mitogens. Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) is a key regulator of this process, making it a prime target for therapeutic intervention. Several drugs targeting VEGFR-2 have been approved and are currently utilized to halt the pathological axis of VEGF-VEGFR. This review will focus on the recent developments in the molecular structure and function of VEGFR-2, the molecular mechanism of VEGFR-2 activation, and its downstream signaling pathway. It will also discuss therapies and experimental drugs approved to inhibit the function of VEGFR-2 and the resistance mechanism.
Introduction
Angiogenesis, the physiological process through which new blood vessels form from pre-existing vessels, plays a crucial role in health and disease. This process is fundamental to the growth and development of organisms, as well as the healing of wounds. However, it also contributes to the progression of various pathological conditions, including cancer, where it facilitates tumor growth and metastasis by supplying nutrients and oxygen. The mechanisms underlying angiogenesis are complex and highly regulated, balancing pro- and anti-angiogenic factors. These mechanisms can be divided into several key steps: activation of endothelial cells (ECs), basement membrane degradation, EC proliferation and migration, tube formation, and, ultimately, the maturation and stabilization of newly formed blood vessels (Shah and Lee 2024).
Activation of endothelial cells is typically initiated by signaling molecules such as VEGF, which binds to receptors on the surface of ECs. This binding initiates a cascade of intracellular signaling that leads to the expression of enzymes capable of degrading the basement membrane, thereby enabling the migration of endothelial cells. The migration and proliferation of endothelial cells are directed toward the source of angiogenic signals. These cells then align to form tubular structures, a process mediated by cell–cell adhesion molecules and extracellular matrix components. Finally, the new vessels mature and stabilize through pericyte recruitment and the deposition of a new basement membrane, ensuring the structural and functional integrity of the developing vasculature. The regulation of angiogenesis is a delicate equilibrium, with an array of growth factors, inhibitors, and environmental conditions influencing the process. Disruptions in this balance can lead to either excessive or insufficient angiogenesis, contributing to the pathogenesis of diseases. Therefore, understanding the mechanisms of angiogenesis is critical for developing therapeutic strategies to modulate angiogenesis in disease treatment and tissue engineering.
VEGF receptors belong to the family of tyrosine kinases that are transmembrane receptors implicated in angiogenesis and lymphangiogenesis. The activity of these receptors is regulated by key signaling molecules known as VEGF mitogens, such as VEGF A-D, which play a crucial role in vascular maintenance, development, and various pathological conditions (Ghalehbandi et al. 2023). There are three types of VEGF receptors, which include VEGFR-1, VEGFR-2, and VEGFR-3. VEGFR-1 has an affinity for VEGF-A and is involved in monocyte migration and hematopoiesis (Kaufman et al. 2021). This receptor also acts as a decoy receptor to control the availability of VEGF ligands and is expressed on the surface of monocytes, macrophages, and endothelial cells (Weddell et al. 2018). VEGFR-2 demonstrates robust tyrosine kinase activity, serving as a critical regulator of VEGF-induced angiogenesis. It is predominantly localized on the surface of endothelial cells, where it facilitates key signaling processes essential for vascular development (Shaik et al. 2020). It has a strong affinity for VEGF-A and promotes endothelial cell migration, proliferation, differentiation, and survival. On the other hand, VEGFR-3 is mainly expressed in lymphatic endothelial cells and has a strong affinity for VEGF-C and VEGF-D. It is important for lymphangiogenesis during embryonic development and is also involved in the regulation of vascular integrity and some pathological conditions, such as tumor-associated lymphangiogenesis (Korhonen et al. 2022).
However, this review article aims to summarize the molecular structure and function of VEGFR, with a particular focus on VEGFR-2, its molecular activation, and the signaling pathways it mediates. Additionally, it discusses the role of VEGFR-2 in the pathophysiology of angiogenesis-related diseases, therapies approved for treating VEGFR-2-regulated pathological angiogenesis, and associated resistance mechanisms.
Structure and function of VEGFR-2
VEGFR is an essential receptor tyrosine kinase implicated in regulating angiogenesis and vasculogenesis. This receptor is a central part of the VEGF-VEGFR system that is crucial to both pathological and physiological angiogenesis and has been explored in biomedical research and the development of therapeutics. The structure and function of VEGFR-2 have been highlighted below:
Molecular characteristics of VEGFR-2
The kinase insert domain receptor (KDR) gene is located on chromosome 4q11-12. This gene encodes human VEGFR-2, a transmembrane glycoprotein composed of 1,356 amino acids (Modi and Kulkarni 2022). VEGFR-2 exists in three different forms classified by molecular weight: the glycosylated mature form (230 kDa) (Takahashi and Shibuya 1997), the non-glycosylated form (150 kDa), and an intermediate form (200 kDa) (Shen et al. 1998). However, only the mature glycosylated form is implicated in intracellular signal transduction. In contrast, other forms are either less active or in the process of becoming fully active, as N-glycosylation is necessary for the receptor’s folding, stability, and cell surface expression (Chandler et al. 2019). In mice, the structure of VEGFR-2 consists of 1,367 amino acids and exists in three structural forms with molecular weights of 180 kDa, 200 kDa, and 220 kDa. It shares 83% structural similarity with human VEGFR-2 (Wang et al. 2020). This receptor is mainly found on the surface of vascular endothelial cells, lymphatic endothelial cells, and embryonic precursor cells, including megakaryocytes, and hematopoietic stem cells (Katoh et al. 1995; Holmes et al. 2007). VEGFR-2 binds to ligands such as VEGF-A, VEGF-C, and VEGF-D, which activate the receptor to mediate the proliferation, invasion, migration, and survival of endothelial cells, while also promoting neovascularization and increasing vascular permeability (Katoh et al. 1995).
Molecular structure of VEGFR-2
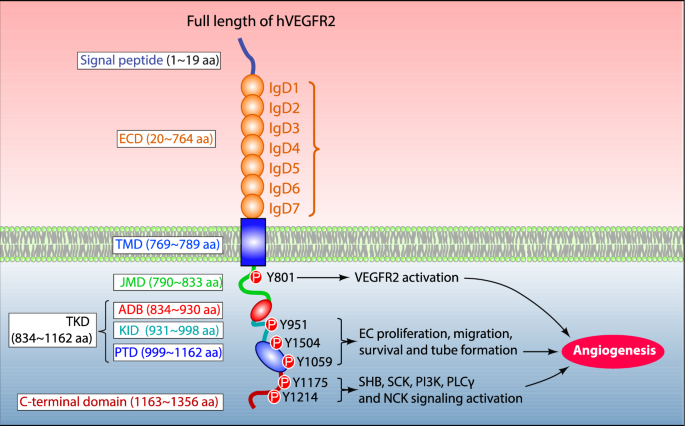
The human VEGFR-2 structure is comprised of 1356 amino acids that are divided into a mature protein (20–1356 aa) and a signal peptide (1–19 aa) (Park et al. 2018; Wang et al. 2020) (Fig. 1). The mature transmembrane protein is further segregated into several structural domains that facilitate its function: A Signal peptide (1–19 aa), extracellular domain (20–764 aa; ECD), transmembrane domain (765–789 aa; TMD), juxtamembrane domain (790–833 aa; JMD), ATP binding domain (834–930 aa; ADB), kinase insert domain (931–998 aa; KID), phosphotransferase domain (999–1162 aa; PTD) and flexible c-terminal domain (1163–1356 aa) (Wang et al. 2020). The mature human VEGFR-2 protein contains 15 phosphorylation sites, including several substrates and ATP binding sites and 18 N-linked glycosylation sites that are essential for VEGFR-2 post-translational modifications, cellular attachment, protein folding, and activation to modulate VEGFR-2 functions (Croci et al. 2014; Chandler et al. 2019; Chung et al. 2019). (Fig. 1).
The molecular illustration of human VEGFR-2 structure. The VEGFR-2 structure is comprised of a signal peptide, an extracellular domain containing seven immunoglobulin-like subdomains (IgD1-7), transmembrane-, juxtamembrane domain (JMD), a catalytic tyrosine kinase domain composed of ATP binding (ADB)-, kinase insert domain (KID) and phosphotransferase domain (PTD) as well as C-terminal domain. Among these domains, key tyrosine residues are phosphorylated upon binding of VEGF to VEGFR2, such as Tyr801 implicated in VEGFR2 activation. Tyr951, Tyr1504, and Tyr1059 located within KID and PTD, are involved in ECs proliferation, migration, and tube formation. The residues present in the c-terminal domain (Tyr1175 and Tyr1214) activate SHB-SCK-PI3 K, PLCγ and NCK signaling pathways which are essential for angiogenesis
No comments:
Post a Comment