For discussion with your doctor if carotid endarterectomy is proposed. I still don't understand why not just completely close the artery after verifying that the Circle of Willis is complete. But I'm not medically trained so ask your doctor those questions. She should know the answers. My right carotid artery is completely closed and has been for the last 10 years. I have zero cognitive problems and have never passed out from lack of blood to the brain.
Could This Stroke-Prevention Specialist Be the Next Great Growth Stock?
Silk Road Medical (NASDAQ: SILK), an innovative medical device company that came public in April, is on a mission to greatly reduce the risk of stroke and its devastating impact on society. Here's why it has a great chance at succeeding and could mint its shareholders a fortune in the process.
The problem
The vast majority of strokes are classified as "ischemic," which is when blood flow to the brain suddenly becomes blocked. A leading cause of ischemic stroke is carotid artery disease, which is the gradual buildup of plaque in the neck arteries that supply blood to the brain. If that plaque breaks away, it can travel up to the brain and cut off critical blood flow, triggering a stroke.
Image source: Silk Road Medical.
While successful CEAs do reduce the risk of stroke over the long term, the procedure itself is very risky. The surgery can accidentally cause plaque to loosen and travel to the heart or brain, which can trigger a heart attack or stroke. The cranial nerve is also at risk of being damaged during the procedure.
These negatives spurred development of a minimally invasive alternative in the 1990s. Called a transfemoral carotid artery stenting (CAS), this process involves placing a stent over the plaque in the artery to keep it from becoming dislodged. Surgeons gain access to the neck by entering through the leg.
While CAS procedures are much less invasive and avoid many of the surgical risks of a CEA, studies have shown that they aren't nearly as effective at lowering the risk of a stroke. As a result, CAS procedures are only performed in a minority of stroke-prevention cases today.
The best of both worlds
Silk Road Medical believes that it has developed a solution that combines the stroke-reduction benefits of a CEA with the low surgical risk of a CAS. The company calls its new procedure a transcarotid artery revascularization (TCAR).Here's how a TCAR works: A surgeon starts by making a small incision in the patient's neck to directly access the plaque-filled artery. Then a product called the ENROUTE Transcarotid Neuroprotection System taps into the artery and reverses the flow of blood away from the brain. The blood then passes through a mesh screen that collects the loose plaque, and the filtered blood reenters the body through the leg. Once the dangerous plaque is removed, an ENROUTE stent is placed on top of the remaining plaque to keep it in place.
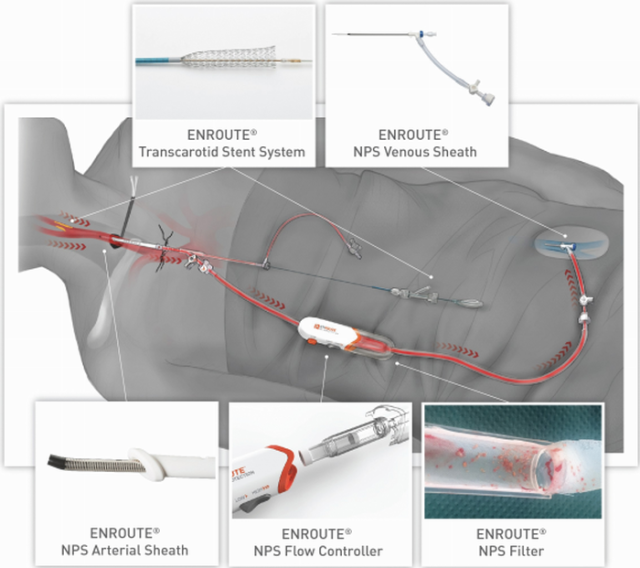
Image source: Silk Road Medical.
Silk Road has the clinical data to prove that the TCAR procedure is superior to the current standard-of-care treatments. The risk of having a stroke in the 30 days following a TCAR procedure is just 1.4%, which compares very favorably to the 2.3% that is observed with a CEA procedure. A TCAR procedure is also faster to perform, reduces the patient's length of stay, and nearly eliminates the risk of cranial nerve injury. The postsurgery scar is also much, much smaller.
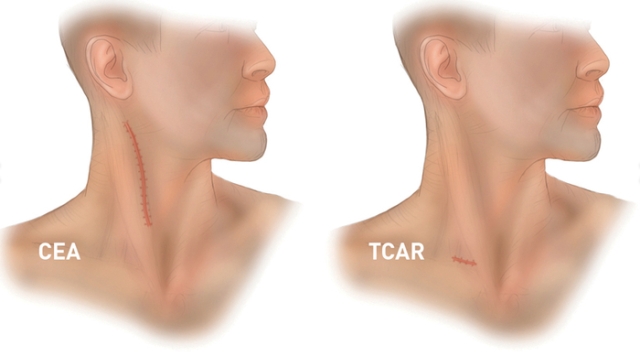
Silk Road's TCAR procedure results in a much smaller neck scar. Image source: Silk Road Medical.
| 2017 | 2018 | 2019 | |
|---|---|---|---|
| Number of TCAR procedures | 1,806 | 4,573 | > 8,000 |
Table source: Silk Road Medical.
The opportunity
About 168,000 carotid revascularization procedures are performed each year in the U.S. Management believes this translates into a $1 billion opportunity, which is a sizable number for a company that is expected to haul in about $62 million in 2019.But that $1 billion figure could be significantly underselling the company's true potential. That's because 4.3 million people in the U.S. have carotid artery disease, and another 427,000 are diagnosed each year. Since the TCAR procedure is minimally invasive and effective, it could help to significantly grow the demand for carotid revascularization procedures. Management believes that the 427,000 new cases each year translates into a $2.6 billion opportunity.
There is also the potential for label expansion claims. Management believes that its technology could also be useful in treating vascular disease of the heart, aortic arch, and brain. Success in any of these additional indications would significantly expand the company's total addressable market opportunity.
Finally, there's also huge
potential for TCAR overseas. Only about 10% of ischemic strokes happen
in the U.S. Silk Road already has secured regulatory approval in Europe,
but it hasn't begun its international commercialization efforts just
yet. The company is actively pursuing regulatory clearance in China and
Japan, too.
No comments:
Post a Comment