Your competent? doctor has had years to figure how to use astrocytes to get you recovered, right? But DID NOTHING, RIGHT? Been fired yet? If not, your board of directors is completely fucking incompetent and has no idea how to run a stroke hospital!
Do you prefer your doctor and hospital incompetence NOT KNOWING? OR NOT DOING?
Astrocytes Regulate Brain State Transitions
- Volume 50, article number 218, (2025)
- Cite this article
29 Accesses
Abstract
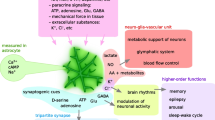
We celebrate the life of our close friend and collaborator, Arne Schousboe, by writing this prose of the role of astrocytes in distinct aspects of arousal. Most animals exhibit cyclic behavioural transitions between sleep and wakefulness. Highly interconnected and complex networks of neurones, which release neurotransmitters, particularly noradrenaline, that target astrocytes by volume transmission, support the arousal system. Astrocyte noradrenergic signalling pathways are intricately connected to energy metabolism, whereby noradrenaline stimulates metabolism and leads to changes in cellular morphology, which is consistent with the maturation, territorial reach and complexity of these glial cells. We briefly discuss historic hypotheses contributing to the ever-going notion that cellular morphology and function affect each other. The message is that astrocytes contribute to sleep-wake transitions through the regulation of homeostatic control; these glial cells are responsible for ionostasis, metabolism, biosynthesis and degradation of neurotransmitters and the regulation of microcirculation and interstitial fluid flow. By regulating brain homeostasis, astrocytes in turn affect neuronal activity in the context of sleep-arousal regulation. “It’s complicated.”® (Arne Schousboe, Denmark).
Foreword
The purpose of this prose is to contribute to the Special Issue celebrating the life and glory of our close friend and collaborator Professor Arne Schousboe (Fig. 1). As our memories have faded on when exactly in the last millennium we get to meet and befriend Arne, we (AV and VP, as MG had not had privilege to meet Arne) will remember, to the last breath we take, his trademark declarative sentence: “It’s complicated.” Namely, we learn most, if not all, we know on metabolism in astrocytes and the brain from Arne. We unequivocally trusted his expertise on the subject. Arne had an incredible ability to explain to us metabolism in terms that we understand, yet challenging us to learn more. We fondly remember our discussions on glutamate and ATP on the crossroads of signalling and metabolism at various places be that on a funicular railway ride to idyllic grounds of Ljubljana Castle in Slovenia or by enjoying a twilight in Heraklion on the island of Crete. Gastronomical pairings on those occasions might have been explorations of our metabolism. Suffice to say, we (AV and VP) commemorated some of our discussions in a triptych of reviews [1,2,3] and by co-editing a book in Advances in Neurobiology with Arne [4]. During these discussions, we learned that metabolism is more complex than our naïve understanding of it at the time. We incorporated metabolism in our lectures on its interface with signalling in and by astrocytes. In a friendly manner, Arne would frequently remind us of additional complexity, which challenged us to learn more. The write up that follows on global states in the brain, which require metabolism and energy to power them. While we did our best to present the subject and refer to metabolic needs, we know very well that “It’s complicated.”® (Arne Schousboe, Denmark).
Similar content being viewed by others
Astrocytes: new evidence, new models, new roles

Astrocytes as the Elements of the Regulation of Higher Brain Functions

Astrocytes of the Brain: Retinue Plays the King
This is a preview of subscription content, log in via an institution to check access

 Ola Bin Shilash
Ola Bin Shilash Hussam Alhathlol
Hussam Alhathlol Rana Alduhaysh
Rana Alduhaysh Razan Almufarriji
Razan Almufarriji Mohammed Bafaquh
Mohammed Bafaquh Yi Cao
Yi Cao Haipeng Deng
Haipeng Deng Shaoyun Liu
Shaoyun Liu Xi Zeng
Xi Zeng Yangyang Gou
Yangyang Gou Weiting Zhang
Weiting Zhang Yixinyuan Li
Yixinyuan Li Hua Yang
Hua Yang Min Peng
Min Peng Weicong Chen
Weicong Chen Chaohua Cui
Chaohua Cui Changsheng Lai
Changsheng Lai