Ask your competent? doctor how many astrocytes died during your stroke and the EXACT PROTOCOLS to recover their functionality. NOTHING from your doctor? You don't have a functioning stroke doctor, why are you seeing them? And your competent? doctor needs to get human testing going in stroke survivors. But is your doctor COMPETENT AT ALL? With nothing on 100% recovery, I'd say not competent.
Astrocyte Transplantations Offer Hope for Treating Brain Disorders
Summary: Astrocytes, critical brain cells, are often lost in neurodegenerative diseases, but recent research highlights the promise of astrocyte transplantation to restore brain function. These transplanted cells integrate into the host brain, forming normal synaptic connections and promoting regeneration, though factors like donor cell type and transplantation timing influence success.
Studies reveal that transplanted astrocytes can survive for up to a year, adapting to their new environment while retaining features of their original region. This emerging therapy offers a promising avenue for treating conditions like ALS, Parkinson’s, and Alzheimer’s.
Key Facts:
- Astrocyte transplantation aids brain regeneration and synaptic function.
- Donor cell type and timing influence integration into the recipient brain.
- Transplanted astrocytes can survive up to a year, matching native astrocytes.
Source: The Conversation
Astrocytes — named for their star-like shape — are a type of brain cell as abundant as neurons in the central nervous system, but little is known about their role in brain health and disease.
Many neurological diseases are caused by or result in the loss of cells in the central nervous system. Some diseases are a result of the loss of specific cells, such as the loss of motor neurons in amyotrophic lateral sclerosis (ALS), the loss of dopaminergic neurons in Parkinson’s disease and the loss of GABAergic neurons in Huntington’s disease.
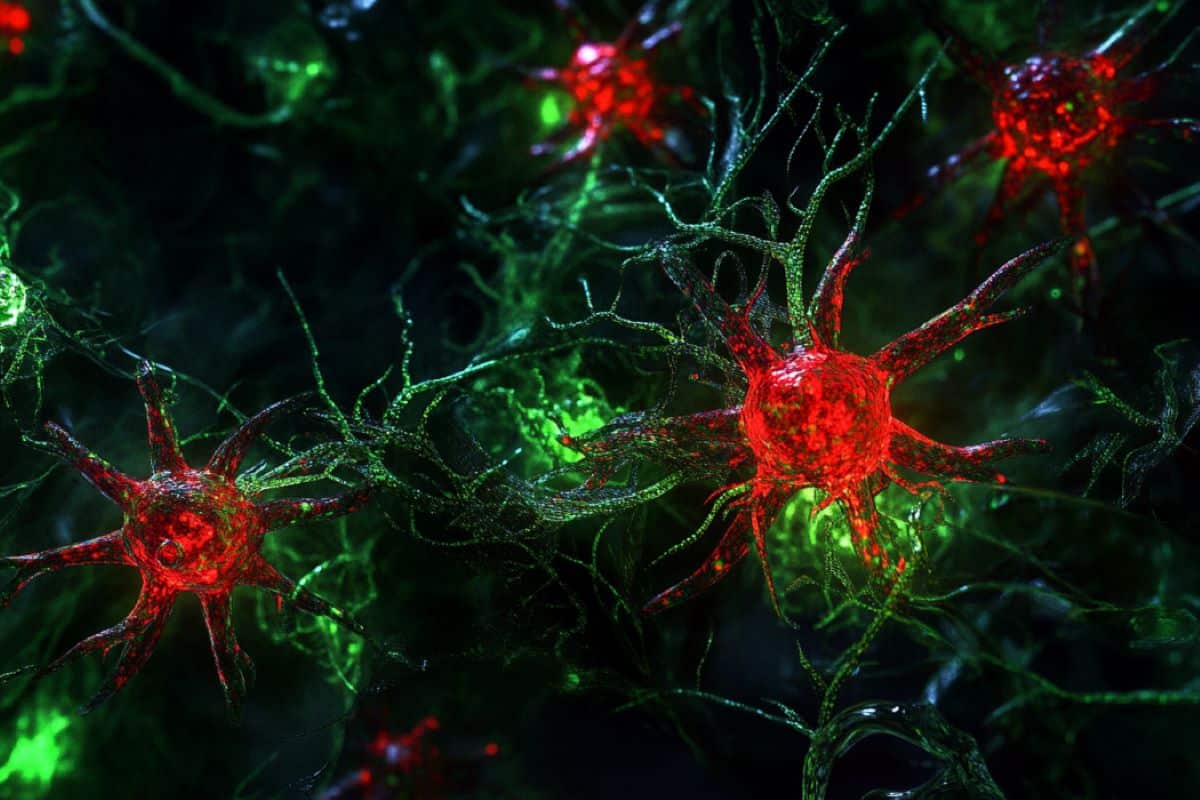
For other neurodegenerative conditions, like Alzheimer’s disease, a key hallmark is the general loss of cells in brain regions responsible for memory formation.
Although many brain diseases are marked by the loss of specific cells, a common link among these diseases is the loss of astrocytes. Interestingly, in some animal studies involving cases such as ALS, introducing disease-causing mutations selectively in astrocytes alone produces ALS symptoms and disease progression.
Transplantation therapy
Emerging evidence indicates that astrocytes take part in major functions of the brain, including homeostasis and neural network modulation that are essential to everyday cognition. A functioning brain requires healthy astrocytes, and finding strategies to heal or replace damaged astrocytes could help in the treatment of neurological diseases.
Cell replacement therapy involves transplanting functional cells in patients. In recent years there have been exciting developments in this area with respect to astrocyte transplantation in animal disease models, with one approach even moving to early clinical trial in ALS patients. While there have been some promising outcomes, treatment success varies from one study to the other.
Our recent study, published in The Journal of Neuroscience, examines how transplanted astrocyte integrate into the recipient central nervous system. We studied the types of transplanted astrocytes, timing of treatment and routes of transplantation.
Preparing astrocytes
First, we prepared astrocyte cultures in petri dishes by extracting immature astrocytes from the cerebral cortex of newborn mice and expanding the cell population.
To track the development of transplanted astrocytes following their delivery to recipient mice, we used astrocytes from genetically modified mice in which astrocytes glow red, and they are transplanted into the brain of mice where astrocytes glow green.
We found that the transplanted astrocytes could survive for up to one year after transplantation, developing normally and integrating into the recipient brain just like the native astrocytes, with just minor differences.
Astrocytes depend on their capability to sense signals and exchange materials within the brain environment through molecules such as receptors and ion channels located on their cell surface.
Transplanted astrocytes displayed comparable numbers of such receptors and channels and possessed similar sizes and complexity when compared to native astrocytes.
Transplanted astrocytes do appear to take some time to catch up to and perfectly match astrocytes in the recipient mice in terms of the production of these receptors and ion channels.
Source, type and location
Intriguingly, we also found that the integration of transplanted astrocytes into the recipient is affected by the age of the mouse, which reflects the maturity of the cellular environment the astrocytes are transplanted into.
When astrocytes were transplanted to an infant mouse, they could migrate and spread more extensively in the host brain. However, when astrocytes were transplanted into a young adult mouse, they were confined to the site of transplantation.
Astrocytes in different regions of the brain and the spinal cord display very different features. We were interested in seeing how astrocytes from one region of the brain integrated into a different region. Astrocytes prepared from the cerebral cortex ended up developing into cortical astrocytes even when placed into the cerebellum.
Therefore, the source and type of astrocytes being transplanted makes a difference, and this intrinsic programming of astrocytes needs to be considered when thinking about astrocyte replacement therapy.
Exciting potential
In recent years, increasing studies have been conducted to investigate the potential of astrocyte transplantation. Similar to our findings, transplanted astrocytes have been found to form normal contacts with neuronal synapses and are functioning normally. Astrocyte transplantation has also been shown to promote brain plasticity and regeneration following injury and in different animal models of neurological diseases.
Therefore, it presents a promising and exciting strategy to treat neurological diseases. By answering principle questions regarding how transplanted astrocytes integrate in the host, our research can support the development of more effective cell therapies that can improve the quality of life of patients.
About this neurology research news
Author: Albert HiuKa Fok and Sabrina Chierzi
Source: The Conversation
Contact: Albert HiuKa Fok and Sabrina Chierzi – The Conversation
Image: The image is credited to Neuroscience News




 Yuxing Zhang
Yuxing Zhang Xin Zhao
Xin Zhao Ying Zhang1,2,
Ying Zhang1,2,