Well, maybe this caused my dissection instead of my whitewater canoe trip.
I had the cardiovascular fitness of an athlete at the time of my stroke. My fitness caused my stroke. The ability to carry canoe and gear over a 1.5 mile portage and the skills to run 21 miles of wilderness whitewater meant I take chances. Something on that trip caused my right carotid plaque to tear, maybe throwing the 60 lb. canoe on my shoulders, or maybe swimming a fully loaded canoe thru several rapids after capsizing, or maybe just sleeping wrong in the tent.
Effect of heart rate on the hemodynamics in healthy and stenosed carotid arteries
Carotid arteries are the important large-diameter arteries in the human body that bifurcate from the common carotid artery (CCA) into the internal and external carotid arteries. The internal carotid artery (ICA) delivers blood to the brain, while the external carotid artery (ECA) delivers blood to the facial tissues. Branching and curvature inside the carotid arteries yield complex flow structures. Internal carotid stenosis is a severe condition because it can cause an ischemic stroke by cutting off the blood flow to the brain. Chen et al.10 reported the temporal and spatial changes in the axial velocity, secondary velocity, and vortex structures due to variations of the proximal curvature and flare of patient-specific carotid arteries reconstructed using magnetic resonance angiograms (MRA). Nagargoje et al.11 reported the effect of primary and secondary velocities on WSS and OSI, considering different axisymmetric and asymmetric idealized bifurcated arteries. Zalud et al.12 reported that the carotid artery with a higher asymmetric bifurcation angle and lower ICA/CCA diameter ratio shows more pro-atherogenic behavior due to the formation of a larger flow separation zone and higher OSI zone compared to the artery with a symmetric branch and higher ICA/CCA diameter ratio. Nagargoje et al.13 observed significant alterations in flow patterns and WSS distribution due to changes in the location and size of the carotid sinus. They concluded that a person is more susceptible to atherosclerosis when the sinus size is large, and the sinus is away from the bifurcation. Albadawi et al.14 observed that the degree and location of stenosis significantly affect the flow structures, WSS, and OSI in a carotid artery. They reported that the risk factor of plaque rupture and thrombus formation increases in higher degrees of stenosis due to increased WSS. Zhang et al.15 numerically studied flow patterns in carotid arteries under healthy, preoperative, and post-operative conditions at a normal heart rate. They reported that due to carotid endarterectomy, the velocity and WSS at the stenosed zone reduced significantly, which improve the health of endothelial cells at that region. Also, the post-stenotic vortices diminish due to carotid endarterectomy. As a result, it reduces the risk of plaque deposition in the post-stenotic region. However, they also reported that post-operation enlargement of the carotid bulb lumen increases the risk of restenosis due to low-level WSS distribution in that region. Several other researchers8,16,17 also studied flow behavior and WSS distribution in healthy and stenosed carotid arteries.
Here,
is the period of the pulsatile waveform. Asgharzadeh and Borazjani18 noticed that when Wo rises, OSI increases in intracranial aneurysms, although they found that Wo has no significant effect on normalized WSS. Simpson and Leylek19 found that Wo strongly influences blood rheology. They reported that as Wo increases, the apparent viscosity of non-Newtonian Carreau–Yasuda fluid shifts toward the constant Newtonian viscosity, and the disparities between TAWSS and OSI predictions for Newtonian and non-Newtonian Carreau–Yasuda fluids become small. Owais and Usmani20 observed that, as the flow pulsation frequency increases, the flow patterns in a bend artery remain similar, but the flow separation becomes delayed.
The Wo of pulsatile arterial flow increases as the heart rate rises. In healthy adults, increased heart rate prevents atherosclerotic plaque formation by increasing WSS and reducing OSI at atherosclerotic-prone sites. The effects of exercise on heart rate elevation and its potential benefits are well documented for healthy adults.21–24 However, the role of elevated heart rates for patients with severe stenotic blockages is not yet well understood and remains debated. Few authors have reported that physical exercise may have adverse impacts on arterial health in patients with stenosis. Giannoglou et al.25 thoroughly reviewed epidemiological studies on the effects of elevated higher heart rates in coronary arteries with atherosclerosis plaque. They reported that in diseased coronary arteries, elevated heart rates increase the amplitude and frequency of the tensile stress applied to the arterial wall and expand the region exposed to low and oscillatory WSS. All these changes adversely impact arterial health and contribute to atherosclerosis-induced damage. Gordon et al.26 reported abnormal contraction–relaxation of arterial tissues during exercise for diseased adults, along with impairment in vasodilator effectiveness. Rauramaa et al.27 reported that exercise schedules were not significantly beneficial in reducing atherosclerotic progression in the patients. It is also reported that exercise may have contrasting effects among healthy and atherosclerotic adults, and thus, moderate exercise schedules are prescribed for patients with a stroke history.28 However, computational fluid dynamics (CFD) studies on flow patterns and stress levels in atherosclerotic arteries during exercise conditions are not well reported. Sood et al.29 investigated the impact of elevated heart rates on post-stenotic flow structure, WSS, and OSI in a pipe with axisymmetric and asymmetric blockage. As the heart rate increased, they noticed more disturbances in the post-stenotic recirculating zone and higher oscillation in WSS. Song et al.30 found that as the heart rate rises, the spread of the low TAWSS (≤0.4 Pa) decreases after the stenosis in an idealized tortuous stenosed coronary artery. Furthermore, the flow structures become more complex with increased heart rate. Owais et al.31 reported that the increased Wo improve arterial health by decreasing the size of primary vortices, detaining the growth of secondary vortices, and delaying the flow instabilities in idealized simple stenosed bend arteries.
The flexibility of the arterial wall may influence arterial hemodynamics. Lopes et al.32 and Moradicheghamahi et al.33 reported rigid wall overestimates velocity and TAWSS compared to the complaint artery. Lopes et al.32 also reported that wall flexibility does not affect the flow division through ICA and ECA. However, several recent studies10,11,14,24,31,34 consider rigid arterial walls neglecting arterial deformation. In support of this justification, Albadawi et al.14 reported that wall flexibility could be neglected in the carotid arteries as it does not significantly affect the arterial hemodynamic parameters. Also, Shahzad et al.35 observed that the effect of arterial elasticity on wall shear stress is negligible at the physiologically relevant Reynolds number (Re) regimes. It was also evidenced from the works of Kumar et al.36 that the average arterial deformation is small over the physiological regimes of blood pressure. They also showed that the peak WSS and OSI do not significantly alter with increased blood pressure. The stiffness of stenosed arteries increases with age which reduces arterial deformability.37,38 Steinman39 also reported that the non-deformable wall assumption turns out to be acceptable for large arteries. These findings evidence that wall deformation has no significant effect on analyzing the pulsatile flow dynamics in large arteries and, therefore, is not considered further. However, from the above-mentioned discussion, it can be noted that the rigid arterial model may slightly overpredict velocity and TAWSS at elevated heart rates. This is a limitation of the present study, which can be taken as future work.
A large number of previous studies8,10–16,40 focused on understanding the flow physics in diseased carotid arteries at normal heart rate. However, there are some recent studies that explained the flow behavior in stenosed arteries at higher heart rates. However, some of them have considered simplified unbranched arterial models,29,31 whereas few researchers have explored higher heart rate effects in branched coronary arteries, where flow is at a smaller Reynolds number.30,41 In our previous study,42 we reported deterioration in the stress levels in the stenotic carotid artery at elevated heart rates. However, the dynamics of vortex structures and their effects on different hemodynamic parameters (WSS, OSI, and pressure drop) were not reported. This research gap has motivated us to conduct the present research as elevated heart rate during exercise may adversely affect the health of patients having stenosis in the carotid artery. Therefore, in the present work, we investigate the impact of elevated heart rate on the pattern of axial velocity, the magnitude of secondary velocity, and three-dimensional vortex structures at different phases of pulsation cycles in healthy, 30%, and 50% stenosed carotid arteries. The effect of these flow features is related to the TAWSS and OSI patterns and to the pressure drop across the stenotic region of the carotid arteries at different heart rates. Furthermore, we have also tried to bring an electrical analogy for the hemodynamic flows by computing flow resistance using pressure drop and flow rate, which is one of the novelties of the present work. In contrast to the previously reported studies, we bring out the consequences of the details of the spatiotemporal variations in the flow pattern on the resulting hemodynamic features, by analyzing a model system having simultaneous geometric, rhythmic, and rheological similitude with physiological systems of individual human subjects. This, in turn, offers a rationalized fundamental basis for deriving patient-specific personalized insight on cardiovascular health. Our study may provide a complementary analytical tool to clinicians in addition to their experience and evidence-based findings from other supportive metadata and established diagnostic procedures.
II. COMPUTATIONAL METHODOLOGY
A. Computational domain
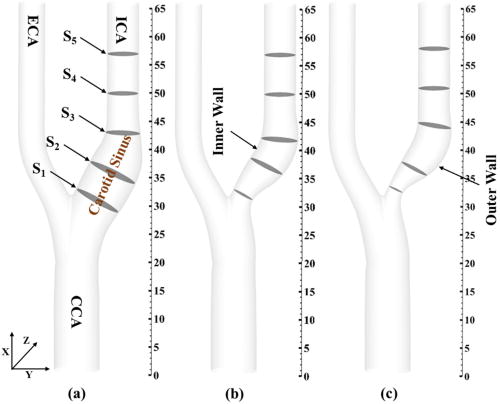
We consider three carotid artery models: one healthy and two with, respectively, 30% and 50% maximum diametric blockage in the ICA, as shown in Fig. 1. The geometrical attributes of healthy, 30%, and 50% eccentric stenotic carotid arteries are taken from Smith et al.43 All these geometries are constructed in SolidWorksTM. The diameter of the common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) are 8, 5.6, and 4.64 mm, respectively.

No comments:
Post a Comment