But you didn't even ask and answer the most important question! Did they get to 100% recovery; the only goal in stroke? No measurement of that, you did useless research. You'll want stroke solved to 100% recovery before you are the 1 in 4 per WHO that has a stroke.
“What's measured, improves.” So said management legend and author Peter F. Drucker
The latest here:
Unsupervised robot-assisted rehabilitation after stroke: feasibility, effect on therapy dose, and user experience
Journal of NeuroEngineering and Rehabilitation volume 21, Article number: 52 (2024)
Abstract
Background
Unsupervised robot-assisted rehabilitation is a promising approach to increase the dose of therapy after stroke, which may help promote sensorimotor recovery without requiring significant additional resources and manpower. However, the unsupervised use of robotic technologies is not yet a standard, as rehabilitation robots often show low usability or are considered unsafe to be used by patients independently. In this paper we explore the feasibility of unsupervised therapy with an upper limb rehabilitation robot in a clinical setting, evaluate the effect on the overall therapy dose, and assess user experience during unsupervised use of the robot and its usability.
Methods
Subacute stroke patients underwent a four-week protocol composed of daily 45 min-sessions of robot-assisted therapy. The first week consisted of supervised therapy, where a therapist explained how to interact with the device. The second week was minimally supervised, i.e., the therapist was present but intervened only if needed. After this phase, if participants learnt how to use the device, they proceeded to two weeks of fully unsupervised training. Feasibility, dose of robot-assisted therapy achieved during unsupervised use, user experience, and usability of the device were evaluated. Questionnaires to evaluate usability and user experience were performed after the minimally supervised week and at the end of the study, to evaluate the impact of therapists’ absence.
Results
Unsupervised robot-assisted therapy was found to be feasible, as 12 out of the 13 recruited participants could progress to unsupervised training. During the two weeks of unsupervised therapy participants on average performed an additional 360 min of robot-assisted rehabilitation. Participants were satisfied with the device usability (mean System Usability Scale scores > 79), and no adverse events or device deficiencies occurred.
Conclusions
We demonstrated that unsupervised robot-assisted therapy in a clinical setting with an actuated device for the upper limb was feasible and can lead to a meaningful increase in therapy dose. These results support the application of unsupervised robot-assisted therapy as a complement to usual care in clinical settings and pave the way to its application in home settings.
Trial registration
Registered on 13.05.2020 on clinicaltrials.gov (NCT04388891).
Background
Stroke often leads to long-term upper limb impairments [1], which may limit stroke survivors during activities of daily living and negatively impact their independence and quality of life.
Therapy can promote recovery and there is growing evidence for therapy dose being a relevant factor influencing sensorimotor recovery, with a higher dose of upper limb therapy contributing to better functional outcomes, even in the chronic phase of stroke [2,3,4]. Current rehabilitation programs are mainly based on supervised one-to-one therapy sessions. Thus, an increase in the dose of therapy for stroke patients, without decreasing its quality, is strongly limited by factors such as low therapist to patient ratios [5] and high rehabilitation-related costs of supervised therapy.
Unsupervised robot-assisted rehabilitation, namely patients training with rehabilitation robots without the supervision or intervention of any external person, bears the potential for increasing therapy dose without significantly weighing on the healthcare system [6]. Rehabilitation robots, here intended as actuated devices that are computer controlled, can actively support movements. Compared to sensor- or VR-based technologies, this allows them to train on a wider range of impairment and makes them more suitable for patients with more severe motor deficits who require active assistance during movements. Furthermore, rehabilitation robots can objectively measure metrics related to sensorimotor ability and reproduce a variety of tasks normally performed by therapists. These features would allow rehabilitation robots to monitor progress throughout a therapy program, adapt the exercises accordingly, as well as provide feedback on performance and progress.
Unfortunately, rehabilitation robots are often complicated to setup and use (i.e., they have low usability), lack the features that would allow automatic adaptation of therapy parameters to the patient state, or might raise concerns in terms of safety. These factors may negatively affect patients’ motivation to train as well as compliance to robot-assisted therapy, and are some of the reasons why a completely unsupervised use of rehabilitation robots has not yet become a standard. The external supervision still required for active devices can take the form of therapists minimally supervising therapy sessions [7], performing remote monitoring [8] or regular meetings to adjust the exercises [9], or caregivers being present to help with using the robot [10]. Therefore, a suitable device and therapy approach to fully exploit the promise of rehabilitation robots to increasing therapy dose without adding significant burden on healthcare practitioners or other external persons remains to be explored.
In this paper, we report on a pilot study investigating the feasibility of unsupervised robot-assisted therapy with a rehabilitation robot for upper limb sensorimotor training, namely the ReHapticKnob [11, 12], in a clinical setting. Subacute stroke patients underwent a four-week protocol, where they progressively transitioned from supervised (i.e., therapist present) to unsupervised (i.e., independent) use of the rehabilitation robot. In that last phase, the robot was freely accessible and no external intervention nor supervision occurred to help interacting with the device or monitor and adapt therapy. The primary goals of this study were to (i) evaluate the feasibility of this approach, (ii) investigate the effect of unsupervised robot-assisted rehabilitation on the overall therapy dose, as well as (iii) assess user experience during unsupervised use of the robot and its usability. The secondary objective was to identify factors (e.g., age, cognitive scores) potentially influencing the feasibility and the achieved dose of unsupervised rehabilitation.
This work is important as it may help establish unsupervised robot-assisted therapy as a feasible and safe method to increase therapy dose with minimal additional workload on any external person, therefore maximizing the efficiency of robot-assisted rehabilitation and opening the door for its application in home settings.
Methods
The ReHapticKnob
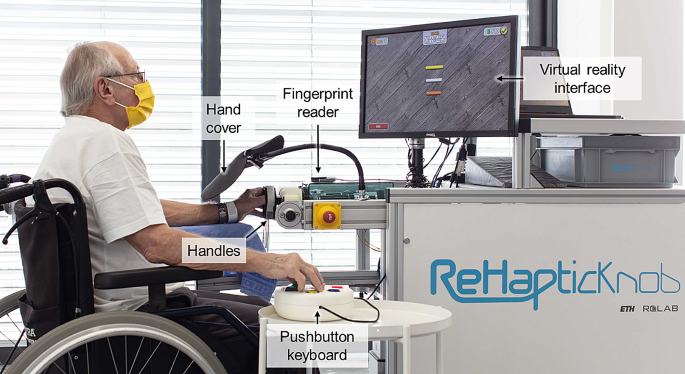
The ReHapticKnob [11, 12] is an end-effector device for sensorimotor rehabilitation of the hand and forearm after stroke (Fig. 1). In previous clinical trials, therapy assisted by the ReHapticKnob and supervised by a therapist was shown to be equivalent (i.e., non-inferior) to carefully dose-matched conventional therapy [13].
A set of seven therapy exercises implemented on this device are based on the neurocognitive therapy concept, which focuses on the integration of motor, sensory, and cognitive functions when performing a task [12, 14]. The exercises focus on the passive or active training of grasping or forearm pronosupination and target subjects with different levels of impairments. The tasks subjects must perform during the exercises include, for example, interacting with virtual objects with different mechanical properties (e.g., different length or different stiffness), memorizing these, and later identifying them based exclusively on the somatosensory input from the impaired limb. In this case, a correct answer corresponds to the correct identification of the object. More details on all tasks and exercises can be found in [12, 14]. Furthermore, each exercise follows an assessment-driven concept, meaning that the initial difficulty level is tailored to the results of specific assessments performed with the ReHapticKnob before the start of the training (for more details see [15]).
To address the challenges raised by unsupervised use, a major focus was placed on improving the usability of the robot, including pilot evaluations with stroke patients and therapists [12]. For example, the graphical user interface was redesigned to be more intuitive and pleasant. Furthermore, clinically-inspired algorithms based on the action and decision process usually performed by therapists in a supervised session were implemented, with the objective of automatically monitoring, controlling, and adapting the content of therapy sessions [16, 17]. For instance, these algorithms automatically adapt the difficulty of an exercise or add a new, more challenging, exercise based on the performance, and provide feedback to guide users through the therapy sessions. Generally, they further decrease the number of actions that need to be learned to interact with the device, thereby increasing usability, and avoiding the need for a therapist to monitor and adjust therapy content over an extended period of time.
Participant performing a therapy exercise with the ReHapticKnob. To train with the device, participants need to fix their fingers to the handles with Velcro straps and log in to their therapy account with the fingerprint reader. A pushbutton keyboard is used to interact with the device and the virtual environment displayed on the screen. The view of the hand (visual feedback) is blocked by the hand cover, as the exercises require users to focus on the sensory feedback from the affected hand to solve the different tasks
Study protocol
This pilot study was approved by the Swiss notified body regulating the use of medical devices (Swissmedic 102681300) and the cantonal ethics commission of Ticino (CE TI 3577). A detailed description of the study protocol is provided in [18].
In short, a sample of 13 participants was chosen, which was considered large enough for a feasibility study while also taking into account a similar group size and drop-out rate (around 20%) compared to our previous studies with the ReHapticKnob [13]. Participants were recruited from the stroke inpatients of the Clinica Hildebrand Centro di riabilitazione Brissago. Inclusion criteria were age between 18 and 90 years old, inclusion within 6 weeks from stroke onset, pre-stroke modified Rankin score [19] ≤ 1, National Institutes of Health Stroke Scale (NIHSS) [20] ≥ 1 in at least one of the items regarding motor or sensory function and ataxia, and signed informed consent form. Exclusion criteria were moderate to severe aphasia (Goodglass-Kaplan’s scale [21] < 3), moderate to severe cognitive deficits (levels of cognitive functioning-revised (LCF-R) [22] < 8), functional impairment of the upper limb due to other pathologies, severe pain in the affected arm (visual analogue scale for pain (VASp) ≥ 5), other pathologies possibly interfering with the study, pacemakers and other active implants, and modified Ashworth Scale [23] > 2 for one or more of the following muscles: shoulder adductors, forearm pronator and supinator, flexors and extensors of elbow, wrist, and fingers.
In order to teach participants to confidently use the device in an unsupervised manner and reduce the risk for adverse events, we specifically designed a systematic protocol for the progressive transition from supervised to unsupervised use of the device [18].
For each participant, the study protocol lasted four weeks. The first week consisted of 5 sessions of 45 min of supervised therapy, where a therapist was present to explain how to use the device and perform the different exercises. The second week consisted of 5 sessions of 45 min of minimally supervised therapy, i.e., participants tried to perform the therapy session independently, but a therapist was still in the room. Despite being present, in this phase the therapist remained in the background and intervened solely upon participant’s request or when necessary (e.g., for safety reasons). At the end of the minimally supervised week, the therapist evaluated the participant’s readiness to continue to the unsupervised phase as well as independence with respect to mobility (e.g., ability to independently access the device) with a custom-made checklist. If participants reached all the goals on the checklist, they proceeded to two weeks of fully unsupervised training. In the unsupervised phase, the device was kept turned on in a freely accessible room in the clinic, and participants could train during a 45-minute timeslot indicated on their daily schedule (business days only), as well as in their free time, evenings, and weekends (the latter not indicated on their schedule). Although for business days a timeslot was booked on their daily schedule to avoid interfering with the conventional therapy plan and to ensure device availability, it is important to note that participants were clearly told that therapy with the ReHapticKnob was voluntary, and no recommendations were given on the daily therapy dose to achieve. Access to the robot was also not monitored nor directly encouraged during the unsupervised phase. If, at the end of the minimally supervised week, a participant was deemed not ready to safely train unsupervised, an additional week of minimally supervised therapy was added. At the end of this second minimally supervised week, the checklist was repeated and if the requirements were met, the participant could train unsupervised for the final week. If not, a third week of minimally supervised therapy was performed.
During each phase, all the robot-assisted therapy sessions were an addition to the conventional therapy plan of the participants (usual care). Participants with very limited mobility (i.e., unable to move on their own from their room to the various therapy stations) were accompanied to the ReHapticKnob upon request by clinical staff dedicated to escorting patients to the various rooms, as for any other conventional therapy. These staff were not trained in the use of the ReHapticKnob, so while they could assist patients in positioning themselves in front of the robot, they were not allowed to help them in interacting with it.
At the beginning and at the end of the study, clinical assessments were performed. Questionnaires to evaluate usability and user experience were performed after the first week of minimally supervised therapy (Usability 1) and at the end of the study (Usability 2), to evaluate the change in perceived usability due to therapists’ absence during the robot-assisted therapy sessions. User experience was further evaluated at the end of each therapy session with the ReHapticKnob by automatically presenting the question “How was your therapy session today?”, to which subjects could answer with a 5-point Visual Analogue Scale represented by different emoticons (VAS – Smiles).
More at link.
No comments:
Post a Comment