But why go thru all the trouble of stem
cells if exosomes are the reason for the benefits? Which must be why no
one seems to be monitoring stem cell survival.
Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations
The latest here:
The role of mesenchymal stem cell transplantation for ischemic stroke and recent research developments
- Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Ischemic stroke is a common cerebrovascular disease that seriously affects human health. However, most patients do not practice self-care and cannot rely on the current clinical treatment for guaranteed functional recovery. Stem cell transplantation is an emerging treatment studied in various central nervous system diseases. More importantly, animal studies show that transplantation of mesenchymal stem cells (MSCs) can alleviate neurological deficits and bring hope to patients suffering from ischemic stroke. This paper reviews the biological characteristics of MSCs and discusses the mechanism and progression of MSC transplantation to provide new therapeutic directions for ischemic stroke.
Introduction
Stroke is a common neurological disease affecting human survival and health; it is characterized by high morbidity, mortality, disability, and a high recurrence rate (1). Statistically, more than 13.7 million people suffer from strokes worldwide annually, and 5.8 million die (2). More remarkable, ischemic stroke incidence is increasing yearly due to the aging population and other reasons. Therefore, ischemic stroke has received increasing attention as the most common type (accounting for ~70% of strokes) (3).
Ischemic stroke is a pathological process caused by a blood circulatory disorder in the brain that leads to neuronal cell death or softening of the brain tissue. As a terminally differentiated cell, the death of a large number of neuronal cells leads to irreversible damage to brain tissue. Early recovery of blood volume in the ischemic area and reduction of nerve cell death are the key points in the treatment of ischemic stroke. However, treatments such as thrombolysis and mechanical thrombectomy benefit only 5% of patients because of narrow therapeutic windows and severe treatment complications (4–6). Thus, further research for safer and more effective ways is still warranted (7).
Stem cells are primitive and unspecialized cells that can develop into diverse specialized cells through mitosis and differentiation and have the potential to regenerate a variety of tissues and organs (8). Extensive preclinical evidence suggests that stem cell transplantation therapy can alleviate brain tissue damage by directional proliferation and differentiation of nerve cells and other pathways. A large number of abnormal nerve cell deaths can occur after an ischemic stroke, and stem cell transplantation will be a viable treatment to relieve neurological deficits in the future (9).
Various types of stem cells have been studied in animal models or clinical studies, such as neural progenitor cells (NPC), mesenchymal stem cells (MSC), endothelial progenitor cells (EPC), and human umbilical cord blood cells (HUCBCs) (10). However, these kinds of stem cells all have limitations in therapeutic effects. For example, EPC therapy faces ethical problems (11). NPCs are tricky to harvest and have a low proliferation rate (12). The treatment of engineered cells, such as induced pluripotent stem cells (iPSC), NT2N cells, CTX0E3, and SB623, is hampered by technology (13, 14). Nevertheless, it is worth noting that MSC cells have become the preferred cells for treating ischemic stroke due to their characteristics, such as high availability, efficient isolating and culturing, high immune tolerance, and fewer treatment complications. Furthermore, MSC cell therapy is not contrary to social ethics (15, 16).
In this paper, we analyze the biological characteristics of MSCs and the neuroprotective mechanism in treating ischemic stroke with the hope of providing new therapeutic directions for ischemic stroke.
Overview of MSCs
MSCs were first described as spindle-bone marrow stromal cells adhered to plastic by Friedenstein and his colleagues in 1970 (17). Four years later, they found that MSCs can form colonies outside the body that adhere to the wall like fibroblasts. Hence, MSCs are also known as cluster unit fibroblasts (CFU-Fs) (18). In 1991, Caplan coined the term “mesenchymal stem cells” and predicted that these mesodermally derived cells would represent the main arsenal of autologous therapies for regenerative purposes (19). With their development in recent decades, MSCs have become the most widely studied stem cell population. They are widely used in clinical trials and/or the treatment of various diseases, especially neurological diseases (20, 21).
MSCs were isolated from bone marrow for the first time. MSCs have previously been isolated from a variety of tissues, such as the lung, liver, kidney, placenta, fallopian tubes, endometrial polyps, adipose tissue, dental pulp, salivary glands, inferior turbinate, umbilical cord blood, menstrual blood, and other tissues (22, 23). They are plastic-adherent and can express mesenchymal markers, including CD90, CD105, CD73, and others, but cannot express CD11b, CD14, CD19, CD34, CD45, and human leukocyte antigen (HLA)-DR (24). MSCs can be harvested from different tissues, and various donor characteristics restrict the surface markers, quality, and isolated numbers of MSCs. Currently, the most frequently reported sources of MSCs utilized in clinical trials are the bone marrow, adipose tissue, and umbilical cord. MSCs obtained from adipose tissue (AD-MSCs) can express CD49d and produce more HGF and VEGF than bone marrow-derived stem cells (BM-MSCs) (25). Compared with bone marrow-derived stem cells, the number of cells obtained from 1 g of fat tissue may be 500 times greater than that of the same weight of bone marrow (26). However, BM-MSCs are safer than AD-MSCs because they can promote the proliferation of existing cancer cells, especially breast cancer (27). Both BM-MSCs and AD-MSCs have significant neurotrophic potential to stimulate neurite growth in DRG-neurons despite different growth factors, which further supports the feasibility of MSC-based stroke treatment (28). Recent investigations into the transplantation of human umbilical cord mesenchymal stem cells (hUC-MSCs)in stroke models have displayed favorable results, including a reduction in infarct size, improved functional recovery, and increased expression of several neuroprotective factors (including VEGF and BDNF) (29). Yet, their isolation can be difficult (30). MSCs from other sources, such as canine-derived MSCs (cMSCs), have not obtained sufficient clinical evidence and cannot be directly applied (31) (Table 1).
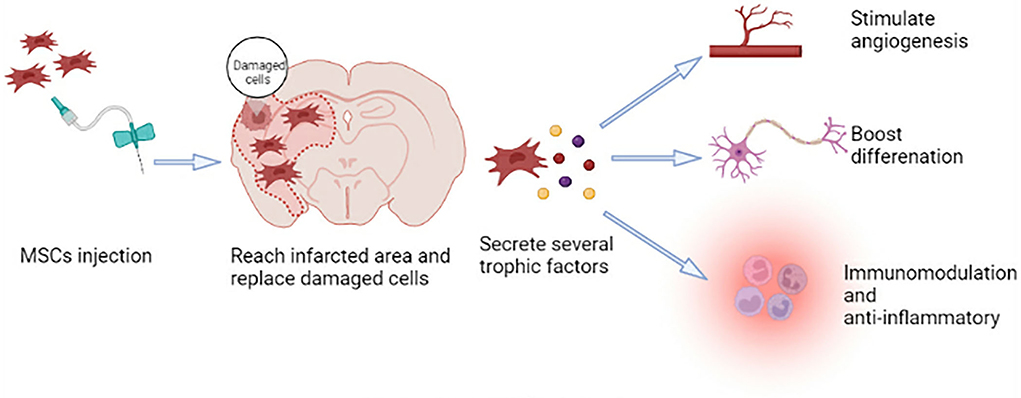
MSCs can self-renew and show polymorphic differentiation (41). They can differentiate into mesoderm cells (described above), endoderm (smooth muscle cells), and ectoderm (neurons) cells under certain conditions (42), which can promote the repair of various damaged tissues (41). Neural regeneration, including neurogenesis, angiogenesis, and synaptic plasticity, is crucial for functional recovery after a stroke. Because MSCs have the characteristics of plasticity, multidirectional differentiation, immunomodulation, and anti-inflammatory, they have the potential to participate in brain regeneration, which can promote tissue repair after ischemic stroke (Figure 1) (43).
The role and research progress of MSC transplantation in the treatment of ischemic stroke
Since Azizi et al. published the first report on the transplantation of human BM-MSCs into the rat brain in 1998, an increasing number of studies on treating neurological diseases by MSC transplantation have been conducted. Moreover, MSC transplantation therapy is gradually shifting from laboratory to clinical therapy. Successful clinical studies demonstrate the clinical transformation of MSC transplantation in treating ischemic stroke. Researchers have adopted various methods (intravenous, artery, and intrathecal injection) to administer MSCs to patients with ischemic stroke. They have focused on the safety, feasibility, and short-term effectiveness of MSCs in treating ischemic stroke. This section summarizes several clinical trials to explore the feasibility of MSCs in treating patients with ischemic stroke.
Plentiful preclinical studies of MSC transplantation therapy in ischemic stroke provide a theoretical basis for clinical practice
Medication, rehabilitation training, and physical union
therapy have not been effective as experimental treatments for ischemic
stroke(So isn't it obvious that you need too get much earlier in the process and stop the 5 causes of the neuronal cascade of death in
the first days, saving millions to billions of neurons? And you can't figure that out?)
. Except for thrombolysis and mechanical thrombectomy, no effective medications or procedures have yet been developed. In this situation, new therapeutic strategies using multiple mechanisms are sought, with MSC transplantation being one of them.
In the preclinical studies, researchers explore different sources of MSCs, feasibility, security, and specific mechanisms of MSC-based therapy. First, using in vitro models, they isolate MSCs from various tissues and demonstrate cell differentiation, neuroprotection, neurogenesis, and angiogenesis. In in vivo models, MSCs are injected into animals by different pathways. Researchers demonstrate that MSC transplantation has a potential therapeutic activity that can repair damaged brain tissue, and it seems feasible and secure (summarized in Table 2).
Model in vitro
The in vitro propagation of MSCs is a three-step process: Extracted from various tissues, MSCs are separated and obtained using density gradient centrifugation digesting culture before being cultured in a plastic cell tissue culture bottle for 3–5 days for further expansion. These steps are then repeated to expand adhered MSCs (63). The following are detailed procedures: To begin, isolate MSCs from multiple tissues such as bone, adipose tissue, tooth tissue, and others using Percoll or Ficoll density gradient centrifugation. Second, rinse MSCs with buffer once to eliminate contaminants before cultivation in 10% fetal bovine serum (FBS) or FBS substitutes, and incubate them in flasks at 37 °C in a humidified 5% CO2 incubator for 2 days. Non-adherent cells are removed by replacing the medium with a fresh one. Subsequently, the attached MSCs proliferate for 2–3 weeks with regular medium change. When the cells have grown to cover about 80% of the flask, it is critical to separate them and allow them to proliferate continually (63).
At present, researchers agree on the multi-lineage differentiation and transplantation potential of MSCs to replace lost tissue after ischemic stroke. When these isolated MSCs are treated with corresponding growth factors or induction medium, they can differentiate into various cell types from different blastoderms (64, 65). These differentiated neuron cells also have functional activity. For example, electrophysiological measurements show that the differentiated cells have voltage-gated sodium and potassium currents, which can be reversibly blocked by tetrodotoxin and tetraethylammonium, respectively (6, 9). However, we need more evidence to prove whether the differentiated cells can fire repetitive action potentials (64).
In addition, MSCs have great neuroprotective effects and promote neurite outgrowth in vitro. Liu et al. claim that BM-MSCs can promote the survival of oxygen-glucose deprivation (OGD) injured neurons, promote axonal outgrowth, and upregulate the expression of GAP-43 when they are cocultured for 48 h with neurons following OGD injury (66). BM-MSCs can also stimulate neurite outgrowth of DRG neurons (67). When hippocampal slices or cortical neurons are cocultured with hMSCs or MSC-derived SB623 cells separated by a semi-porous membrane or with MSC- or SB623 cell-conditioned medium following OGD, neural cell death or damage is decreased. Moreover, 11 neurotrophic factors are identified as secreted by MSCs and/or SB623 cells, and most of them are potentially beneficial to neural tissue following an ischemic insult (68). Furthermore, BM-MSCs from normal healthy and cerebral ischemia rats increase neuronal survival and connectivity in glial-neuron mixed cultures (69). These reports support the fact that MSCs have neuroprotective effects and stimulate neurite development in vitro.
Lastly, the ability of MSCs to stimulate angiogenesis, participate in vascularization, and re-establish a blood supply is evaluated in in vitro models. It is also the fundamental process of tissue repair (70). In in vitro models, MSCs can differentiate into endothelial lineage cells to protect ECs against hypoxia-induced cell death (71), promote the formation of endothelial rings (65), improve the paracrine activity of angiogenic growth factors and EC migration, and form mature vascular tissue (70). Hypoxic preconditioning enhances the pro-angiogenic effects of MSCs by increasing the expression of angiogenic growth factors and boosting the proliferation and migration of ECs (72).
Thus, MSCs show great promise in vitro, including the potential for cell differentiation, neuroprotection, neurogenesis, and pro-angiogenesis. Therefore, several studies have ulteriorly transplanted MSCs into animal models of ischemic stroke and evaluated the outcomes as discussed below.
More at link.
 Li Zhou†,
Li Zhou†,  Jiani Wang
Jiani Wang Jiagui Huang
Jiagui Huang Qin Yang
Qin Yang

No comments:
Post a Comment