Treatments would be much more effective if you stopped the 5 causes of the neuronal cascade of death
in the first week, saving hundreds of millions to billions of neurons.
THAT would make these suggested interventions actually work. Does no one
in the world understand how to solve stroke?
Task-specific training versus usual care to improve upper limb function after stroke: the “Task-AT Home” randomised controlled trial protocol
- 1School of Health Sciences, College of Health, Medicine and Wellbeing, The University of Newcastle, Callaghan, NSW, Australia
- 2Occupational Therapy, School of Allied Health, Human Services and Sport, La Trobe University, Melbourne, VIC, Australia
- 3Brain Research Institute, Florey Institute of Neuroscience and Mental Health, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Melbourne, VIC, Australia
- 4School of Health and Social Wellbeing, Faculty of Health and Applied Sciences, University of the West of England, Bristol, England, United Kingdom
- 5Department of Rehabilitation Medicine, Amsterdam Movement Science and Amsterdam Neuroscience, Amsterdam University Medical Center, Amsterdam, Netherlands
- 6Clinical Research Design, Information Technology and Statistical Support (CReDITSS) Unit, Hunter Medical Research Institute, New Lambton Heights, NSW, Australia
- 7Hunter Medical Research Institute, The University of Newcastle, New Lambton, NSW, Australia
- 8School of Medicine and Public Health, College of Health, Medicine and Wellbeing, The University of Newcastle, Callaghan, NSW, Australia
- 9Nursing Research Institute, St Vincent’s Network Sydney and Australian Catholic University, Sydney, NSW, Australia
Background: Sixty percent of people have non-functional arms 6 months after stroke. More effective treatments are needed. Cochrane Reviews show low-quality evidence that task-specific training improves upper limb function. Our feasibility trial showed 56 h of task-specific training over 6 weeks resulted in an increase of a median 6 points on the Action Research Arm test (ARAT), demonstrating the need for more definitive evidence from a larger randomised controlled trial. Task-AT Home is a two-arm, assessor-blinded, multicentre randomised, controlled study, conducted in the home setting.
Aim: The objective is to determine whether task-specific training is a more effective treatment than usual care, for improving upper limb function, amount of upper limb use, and health related quality of life at 6 weeks and 6 months after intervention commencement. Our primary hypothesis is that upper limb function will achieve a ≥ 5 point improvement on the ARAT in the task-specific training group compared to the usual care group, after 6 weeks of intervention.
Methods: Participants living at home, with remaining upper limb deficit, are recruited at 3 months after stroke from sites in NSW and Victoria, Australia. Following baseline assessment, participants are randomised to 6 weeks of either task-specific or usual care intervention, stratified for upper limb function based on the ARAT score. The task-specific group receive 14 h of therapist-led task-specific training plus 42 h of guided self-practice. The primary outcome measure is the ARAT at 6 weeks. Secondary measures include the Motor Activity Log (MAL) at 6 weeks and the ARAT, MAL and EQ5D-5 L at 6 months. Assessments occur at baseline, after 6 weeks of intervention, and at 6 months after intervention commencement. Analysis will be intention to treat using a generalised linear mixed model to report estimated mean differences in scores between the two groups at each timepoint with 95% confidence interval and value of p.
Discussion: If the task-specific home-based training programme is more effective than usual care in improving arm function, implementation of the programme into clinical practice would potentially lead to improvements in upper limb function and quality of life for people with stroke.
Clinical Trial Registration: ANZCTR.org.au/ACTRN12617001631392p.aspx
Introduction
Background and rationale
Worldwide, 12.2 million people had a stroke in 2019 (1). In Australia, where this study occurs, 1,000 new strokes occur every week and the resultant cost burden is $5 billion a year (2). Problems performing everyday activities of daily living persist 6 months after stroke for 95% of people (3), largely due to not being able to use the arm. Only half of all stroke survivors with an initial affected upper limb regain some useful upper limb function after 6 months (4). Sixty percent of severely impaired and 30% moderately impaired stroke survivors have been reported as having non-functional arms after 6 months (5). Poor recovery of upper limb function is associated with a low health-related quality of life (6, 7).
A Cochrane systematic review of repetitive task training in 2007 (8), most recently updated in 2017 (9), showed a moderate level of evidence for effect of task-specific training on functional ambulation and walking distance, but a low level of evidence for effect on arm and hand function, at ≤6 months after stroke. At the time of conceiving this trial, few studies (10, 11) had directly addressed the hypotheses that task-specific training for the upper limb is more effective than usual care. Several exploratory studies had compared task-specific/functional training to other interventions. For example, Winstein et al. (11) compared 22 participants receiving functional training in addition to current practice to 21 receiving only usual care, and found an advantage for functional training. Other studies compared task-specific training to alternative treatments such as strength training (12), education (13) or Brunnstrom and Bobath technique (14). About half of the existing studies at the time favoured task-specific training but all were small in sample size (N < 53), most had design limitations (e.g., lack of intention to treat analysis, blinded assessor, poor allocation concealment) and several combined the treatment with added interventions so the effect of task-specific training alone could not be estimated. Encouragingly, the EXCITE trial of constraint-induced therapy (CIMT), which contains functional training similar to task-specific training and which was community based, found a positive effect for CIMT on the Wolf Motor Function test at 12 months (primary outcome Wolf Motor Function test) (15).
In a phased approach to developing better treatments, in 2014 we completed a Phase II feasibility RCT with 48 participants (24 task-specific, 23 usual care) using an preliminary version of the design in this protocol (16). Proof-of-concept of task-specific training was demonstrated, with an improvement in median ARAT score (primary outcome measure: range 0–57) of the task-specific group from 8.5 (IQR: 3.0, 24.0) at baseline to 14.5 (3.5, 26.0) at 6 months, compared the usual care group, which had a median score of 4 (3.0, 14.0) which did not change (17). Participants, 85% of whom had moderate–severe impairment, performed a median 157 repetitions per visit plus a median 52 repetitions per day of self-practice, fulfilling the goal of 100–300 reps per day, proving the treatment dose is feasible. 96% of participants rated task-specific training acceptable, 71% rated 1 h of independent practice/day acceptable and 83% reported it improved their arm function. There were no serious adverse events. Based on the results of the feasibility study, we decided to go to the next step to conduct a Phase III trial to investigate effectiveness.
Given that a several existing studies show positive effects of task-specific training compared to other interventions, an important pragmatic question to be answered is whether the new treatment is more beneficial than care routinely delivered now. Consequently, we chose as our comparator group, usual care, where we will not alter treatment intensity or content in the usual care group. Usual care may provide less treatment, but intensity between the two groups will not be matched for the following reasons: (1) matching intensity would not be a true comparison to usual care, because current service provision does not currently offer the proposed intensity of treatment; (2) it is known that increasing intensity of current practice does not deliver the quantum change needed to improve stroke outcome at a population level (18). The importance of our question to health service providers overrides the potential for a difference in intensity of the two treatments. Moreover, if task-specific training is shown to be effective, there will be incentive for increasing intensity when implemented. Treatment intensity will however be carefully recorded.
Since the conception of the present study, in 2016 a randomised controlled trial with 361 participants with stroke, comparing task-oriented rehabilitation to dose-equivalent occupational therapy, or to usual care was published by Winstein et al. (ICARE trial) (19). Findings revealed that a structured, task-oriented rehabilitation program did not significantly improve motor function or recovery beyond either an equivalent or a lower dose of usual care upper extremity rehabilitation. The therapy content was 30 h delivered over 10 weeks and consisted of “intense bouts of task-specific practice, strengthening exercises, shoulder stability/mobility training and motivational enhancements to enable self-confidence and autonomy support to use the stroke-affected arm and hand in valued activities outside the clinic” (19) (Supplementary content, etable2). As the ICARE trial had a similar aim to our study, it is of interest to compare the methods. There are some key differences. We have a more intensive 56 h of intervention over 6 weeks, compared to 30 h over 10 weeks in Winstein 2016 (19). Our study is home-based rather than outpatient based. Primary outcome measure and timepoint also differ. Our primary outcome timepoint (ARAT) is immediately after the intervention at 6 weeks and at 6 months post-randomization. In comparison, in the ICARE trial, the primary outcome (log-transformed Wolf Motor Function Test time score) was measured at 12 months post-randomization. Our study will provide key additional information on the effect of intensity, timing of primary outcome measure and environment. In addition, another small trial was also reported in 2019 (20) comparing task-oriented training to usual care, for the upper limb, with 14 participants allocated to each group. The task-oriented group improved significantly more than usual care on the Wolf Motor Function Test and the Fugl-Meyer upper extremity assessment.
There is a trend to moving inpatient rehabilitation services into the community (21). In Australia in 2020 for example, 42% of services were providing early discharge services compared to 17% in 2016 (22). It follows that much upper limb recovery occurs in the community, after discharge from hospital. At the time of conceiving this study however, a Cochrane review of a range of upper-limb therapies at home (23) showed that we did not yet know if therapy was effective in the home. The likelihood is that home-based treatment will be more beneficial than hospital-based treatment since practice in the person’s own environment is more meaningful (24). In addition, participants might prefer treatment in their home. For example, 96% of participants in our feasibility study (16) found it acceptable. The following two systematic reviews report favourable effects for home rehabilitation in general, though they did not focus particularly on the upper limb. Hillier et al. (25) reported a significant effect in favour of home-based rehabilitation in general compared to centre-based rehabilitation at 6 weeks and 3–6 months post-intervention. Similarly Chi et al. (26) found that home-based rehabilitation led to moderate improvements on physical function in home-dwelling patients with a stroke. To summarise, there are compelling reasons to base therapy at home, but more evidence is still needed.
Study aims and hypotheses
The primary aim of the Task-AT Home study is to determine if a 6-week task-specific home-based training programme for stroke survivors is more effective than usual care in improving upper limb function and amount of arm use.
The hypotheses relating to this aim are as follows:
1. Upper limb function will achieve a ≥ 5 point improvement on the Action Research Arm Test in the task-specific training group compared to the usual care group, immediately after 6 weeks of intervention (primary outcome).
2. Amount of upper limb use in everyday life will achieve a ≥ 1 point improvement on the Motor Activity Log in the task-specific training group compared to the usual care group immediately after 6 weeks of intervention (secondary outcome).
3. The difference in upper limb function on the Action Research Arm Test between groups will persist to the end of follow up at 6 months after intervention commencement (secondary outcome).
There are 2 secondary aims:
1. Secondary aim 1: To determine if a 6-week task-specific home-based training programme for stroke survivors is more effective than usual care in improving health related quality of life.
2. Hypothesis for secondary aim 1: Health related quality of life will achieve a minimally important change of 0.08 point improvement on the EQ5D-5 L in the task-specific training group compared to the usual care group at follow up at 6 months.
3. Secondary aim 2: To identify costs and consequences of both interventions, where consequence is measured by change in health related quality of life.
Supplementary aims:
a. Identify potential barriers and possible solutions to implementation of task-specific training. A qualitative study is being conducted using a narrative inquiry methodology. In-depth interviews with eight stroke survivors, three caregivers and four therapists will capture a broad range of perspectives following participation in the training. A detailed description of this study will be reported elsewhere.
b. Originally a sub-study was included in our plan to determine changes in motor control in response to training, using kinematics of reach-to-grasp., in the task-specific training group compared to the usual care group. However, due to adjustments that needed to be made to recruitment and funding due to Covid-19, resources were instead redirected to execution of the main trial, so this sub-study could not occur.
Methods and analysis
Trial design
This study is a two-arm, assessor-blinded, multicentre randomised controlled trial comparing task-specific treatment to usual care for the arm and hand after stroke. The study will occur in participants’ homes with the interventions delivered by therapists upskilled and employed to deliver the task specific research intervention.
Research personnel responsible for data collection will be blinded to the treatment group to which a participant is assigned. Those involved in the delivery of the treatment will not be blinded. We have also aimed to blind participants as much as possible to group allocation by (a) not informing them deliberately of group allocation – instead they were told they would be visited by a therapist soon and the approximate timeline of this visit. The visit would occur within a week of assessment (if allocated to the task-specific treatment) or in approximately 6 weeks (if allocated to usual care).
The trial is registered with the ANZCTR. Appendix 1 shows key Trial Registration data.
Study population
Participants with stroke affecting upper limb function will represent the target study population. Participants will be identified during their inpatient stay in stroke wards at hospitals, or via community stroke services, in New South Wales (NSW) and Victoria, Australia. In NSW these sites include the Local Health Districts of Hunter New England Health (Kurri Kurri, Maitland, Cessnock and Manning Hospitals, Transitional Aged Care Programme Hunter Valley), Central Coast (Gosford, Long Jetty and Wyong Hospitals, Woy Woy Rehabilitation Centre), Mid North Coast (Port Macquarie, Kempsey and Wauchope Hospitals), Berkley Vale Private Hospital and Mt. Wilga Private Rehabilitation Hospital. In Victoria the sites include health services from Austin Health, Eastern Health and Western Health. Potential participants are identified by therapists working in these organisations and are then screened by the trial manager, at between 2.5 and 3.5 months after stroke. Potential participants who learn about the study via social media or the Hunter Medical Research Institute (HMRI) website or study website are also able to self-refer to the study by contacting the research team directly.
Inclusion criteria are diagnosis of primary or recurrent stroke, including stroke caused by focal cerebral infarction (ischemic stroke), intracerebral haemorrhage, subarachnoid haemorrhage and cerebral venous thrombosis (27); discharged home (i.e., permanent address, may include care home/sheltered accommodation); approximately 3 months post stroke (between 2.5 and 3.5 months post stroke); remaining upper limb movement deficit defined as being unable to pick up a 6 mm ball bearing from the tabletop, between index finger and thumb, and place it on a shelf 37 cm above table (item from Action Research Arm Test); informed written consent. Exclusion criteria are upper limb movement deficits attributable to non-stroke pathology; unable to lift hand off lap at all when asked to place hand behind head; severe fixed contractures of elbow or wrist (i.e., grade 4 on the modified Ashworth scale).
Trial interventions
Task-specific intervention
The task-specific intervention will be guided by a detailed protocol (28). The intervention will occur in the participant’s home. In brief, the intervention therapist analyses the whole of the task which is to be trained, e.g., reach-to-grasp., to identify movement components to be prioritised for training and individual movement performance targets to be reached. This will be necessarily different for each participant. The person’s visual attention is directed to regulatory cues in the environment, which are organised to be functionally relevant, individualised and varied, by providing meaningful everyday objects of different sizes, weight and shape, in different positions. The therapist’s role is like a sports coach. He/she uses knowledge of critical biomechanical characteristics of the task to give instructions (by demonstration or verbally) which are concrete and task oriented. Repetitive practice and motor learning principles are used to empower the participant to practice on their own.
Training will be delivered according to an exercise manual containing 142 exercises in words and photographs including variations of the exercises to allow for different levels of difficulty and complexity.
The manual was developed during our previous feasibility trial by a team of therapists during the feasibility trial (Paulette van Vliet, Ailie Turton, Fredreike van Wijck, Paul Cunningham) (28) with consultation with user representatives and local specialist neurophysiotherapists. It describes the underlying principles, clinical objectives and individual exercises to achieve the stated objectives. It also details a menu of the treatment strategies (in words and pictures) and includes variations of the treatment strategies to allow for different levels of difficulty and complexity. A description of the development of the manual is available (28), however the manual itself is under embargo until the completion of the trial.
Participants receive 14 × 1-h visits from a therapist over the 6 weeks (3 visits in weeks 1–3, 2 visits in weeks 4–5, 1 visit during week 6). This will replace any usual care training for the upper limb. The intensity of practice within each 1-h session will be dependent on individual participant’s capabilities, but high numbers of repetitions will be encouraged, with the aim of delivering between 100 and 300 repetitions within each 1-h session. Beyond the target of 100–300 reps, participants will do as many repetitions as they can accomplish in 1 h, within limits of fatigue. Any repetition is counted, full range is not required, and repetitions are recorded on a task-specific therapy log form. The number of minutes spent practising each exercise is also recorded on the form, as well as the number of the session (out of 14 sessions), and the total duration of task-specific practice in each session (minutes).
Stretches may be indicated when decreased muscle length is present as a secondary consequence from having had a stroke. When decreased muscle length is interfering with performance of exercises from the task-specific exercise manual, a maximum of 5 min during the 1-h treatment may be spent in stretching and may include short duration stretches of between 8 s to 5 min. Longer duration stretches, of 20–30 min duration, may also be prescribed as part of the patient’s self-managed programme, to be performed outside of their 1 h daily self-practice. Long duration stretches will not be used in the one-to-one treatment sessions with the therapist. The stretches that may be used are described with photographs in a document ‘Upper Limb Stretches’, which is given to the therapists.
Self-practice
Participants will be asked to perform in addition to therapy sessions, 1 h/day of self-practice. Adherence is encouraged by joint goal setting, providing a booklet about recovery from stroke emphasising potential for ‘rewiring’ the brain through practice, and using a self-practice log to record repetitions, and the date on which they were performed. The self-practice log is checked by the therapist at the beginning of each therapy session, and the participant is assisted to record the repetitions done if needed. The role of the carer will be to encourage the participant to practice and assist with equipment to enable practice and with recording practice.
Usual care intervention
The control group for this trial will receive ‘usual care’. Usual care will be provided according to the usual provision in the Local health District(s)/health services, by the usual staff (not staff employed on the trial). In usual care the frequency and content of therapy is variable according to the individual’s pathway and the range of community services available. Community rehabilitation services may be delivered via early supported discharge, day hospital, community-based rehabilitation provided in the home or outpatient rehabilitation (29). Usual care variously consists of facilitation of muscle activity, strengthening exercises, soft tissue and joint mobilisation, positioning, training of sensation, and education (30). While it may include practice of everyday functions, it does not include systematic practice of part-tasks, biomechanical analysis, engaging environmental cues or high numbers of repetitions. Usual care therapists will be requested to indicate any treatment they used for an identified participant on a checklist form listing usual care treatment activities called the Upper Limb Usual Care Therapy Log.
Participants allocated to the usual care group will be provided with a booklet about recovery after stroke, with information about details of the frequency of assessment visits rather than about task-specific training. At the end of a participant’s involvement in the trial, at 6 months after recruitment, the usual care participants will be offered a one-off consultation with a research therapist, in which several exercises from the task-specific manual will be recommended.
Who delivers treatment, amount of treatment and adherence
Different therapists will deliver the usual care and task-specific interventions. Usual care will be delivered by the usual clinical service physiotherapists and/or occupational therapists. Task-specific intervention will be delivered by a group of therapists employed to deliver the intervention in the trial. Intervention therapists will be trained to deliver task-specific training over a 2-day course including theory, and practice with participants with stroke, prior to delivering the treatment. Further training sessions with participants with stroke, and/or follow-up discussion about intervention, with the trainer are available, if needed. Therapists will also receive instruction in the importance of strictly following the treatment manual.
The treatment duration for the task-specific intervention will be 6 weeks, with 1-h visits occurring 3 times in the first 3 weeks, twice in each of the next 2 weeks, then once in the final week, (tapered to increase self-management).
For each intervention therapist, fidelity to treatment protocol will be assessed. For each therapist, we will aim to assess fidelity on 4 separate occasions. A treatment fidelity checklist will be used. If fidelity to treatment schedule is <90% then further training will be given within the 2 weeks following the fidelity assessment, until the 90% criteria is achieved.
Outcomes
All outcome measures are performed immediately after the 6 weeks of intervention and at 6 months after intervention commencement by an assessor blind to group allocation. The primary outcome measure is a test of arm function and impairment, the Action Research Arm Test (ARAT) (31), immediately after the 6 weeks of intervention. It consists of 19 items focusing on grasping objects of different shapes and sizes, and gross arm movements. Each item is given an ordinal score of 0, 1, 2, or 3 with higher values indicating better function. The test has high inter-rater and test–retest reliability, good validity and is sensitive to therapy-related changes after stroke. A standardized test protocol will be followed (32).
Secondary outcome measures are (a) the Motor Activity Log (33); (b) the ARAT at 6 months to indicate whether changes occurring at end of 6 weeks are sustained in the longer term; and (c) the EQ5D-5 L (34). The Motor Activity Log is a self-report of quality of upper limb movement and amount of use, to capture the patient perspective. A clinically important mean change on the Motor Activity Log is ≥1; (scale range 0–5). This is a valid, reliable and responsive tool and its scores are strongly correlated with arm accelerometry data (33). The EQ5D-5 L is a standardised measure of health status providing a simple, generic measure of health for clinical and economic appraisal (34). It includes a descriptive system comprising 5 dimensions - mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels: no problems, slight problems, moderate problems, severe problems, and extreme problems. It also includes a Visual Analogue scale which records the respondent’s self-rated health on a 20 cm vertical, visual analogue scale with endpoints labelled ‘the best health you can imagine’ and ‘the worst health you can imagine’.
Tertiary outcomes are (a) the Wolf Motor Function test (WMFT) (35); (b) the Fugl-Meyer assessment (upper limb section) (36); (c) the Caregiver Strain Index; and (d) the modified Rankin Scale. The Wolf Motor Function Test assesses a wide range of functional abilities of the upper limb. Fifteen movements both with and without objects are measured according to quality of task performance (graded 0–5) and the time to complete the task. In addition, the number of tasks that can be performed in less than 120 s is assessed. The test is responsive to measuring changes in our target group (15), has high inter-rater reliability, test–retest reliability, internal consistency, and construct validity (35, 37).
The upper limb section of the Fugl-Meyer assessment (FM-UL) (36, 38) is also included as this assessment has been identified by consensus at the Stroke Recovery and Rehabilitation Roundtable (39) as a core measure of sensorimotor recovery that should be included in stroke trials, to allow future pooling of participant data across studies and institutions aiding meta-analyses of completed trials. Caregiver burden will be assessed using the Caregiver Strain Index (40). The CSI is included as we would expect carer burden to decrease with improved upper limb function. The Modified Rankin Scale will be used to measure degree of disability or dependence (41). Finally, to allow determination of costs associated with treatments, the Client Services Receipt Inventory will be used (42).
The time schedule of enrolment, interventions and assessments, is shown in Figure 1.
Participant recruitment
Potential participants may be in hospital awaiting discharge or they may already be at home following stroke. In both situations the recruitment procedure will take place over at least two occasions. Screening will take place before consent. First, potential participants will be identified by a clinician, who will check whether potential participants meet the eligibility criteria. If potential participants meet the eligibility criteria, the clinician will make the initial approach to the potential participant, and give the person an invitation letter and Patient Information Sheet (PIS) [approved by the local Research Ethics Committee (REC)]. The clinician will ask the person’s permission to pass their contact details onto a member of the research team. The participant will have at least 24–48 h to read the PIS and to discuss their participation with others outside the research team (e.g., relatives or friends) if they wish. The researcher will go through the PIS and explain the project to make sure the person understands the nature and intensity of the interventions, the randomisation procedure and a participant’s right to withdraw at any time without compromising their care and research governance issues. The researcher will answer questions, confirm the participant’s eligibility and take written informed consent if the participant decides to participate. Those who consent to participate will be assessed at baseline. Following this, participants will be randomised, and the Trial Manager will contact participants by telephone to inform them when their next assessment will be (usual care group) or organise their first therapy appointment and provide the therapist name (task-specific training group). Details of all participants approached for the trial and reason(s) for non-participation (e.g., reason for being ineligible or participant refusal) will be documented.
Strategies being employed for achieving adequate participant enrolment include (a) increasing the number of sites; (b) increased phone and email communication; (c) prompt resolving of local issues relating to recruitment challenges; (d) visiting the sites when possible, and (e) sending a newsletter with news about the trial.
More at link.
 Paulette van Vliet
Paulette van Vliet Leeanne Mary Carey
Leeanne Mary Carey Ailie Turton
Ailie Turton Gert Kwakkel
Gert Kwakkel Kerrin Palazzi6,
Kerrin Palazzi6,  Heidi Lavis
Heidi Lavis Sandy Middleton
Sandy Middleton Bleydy Dimech-Betancourt
Bleydy Dimech-Betancourt Meredith Tavener
Meredith Tavener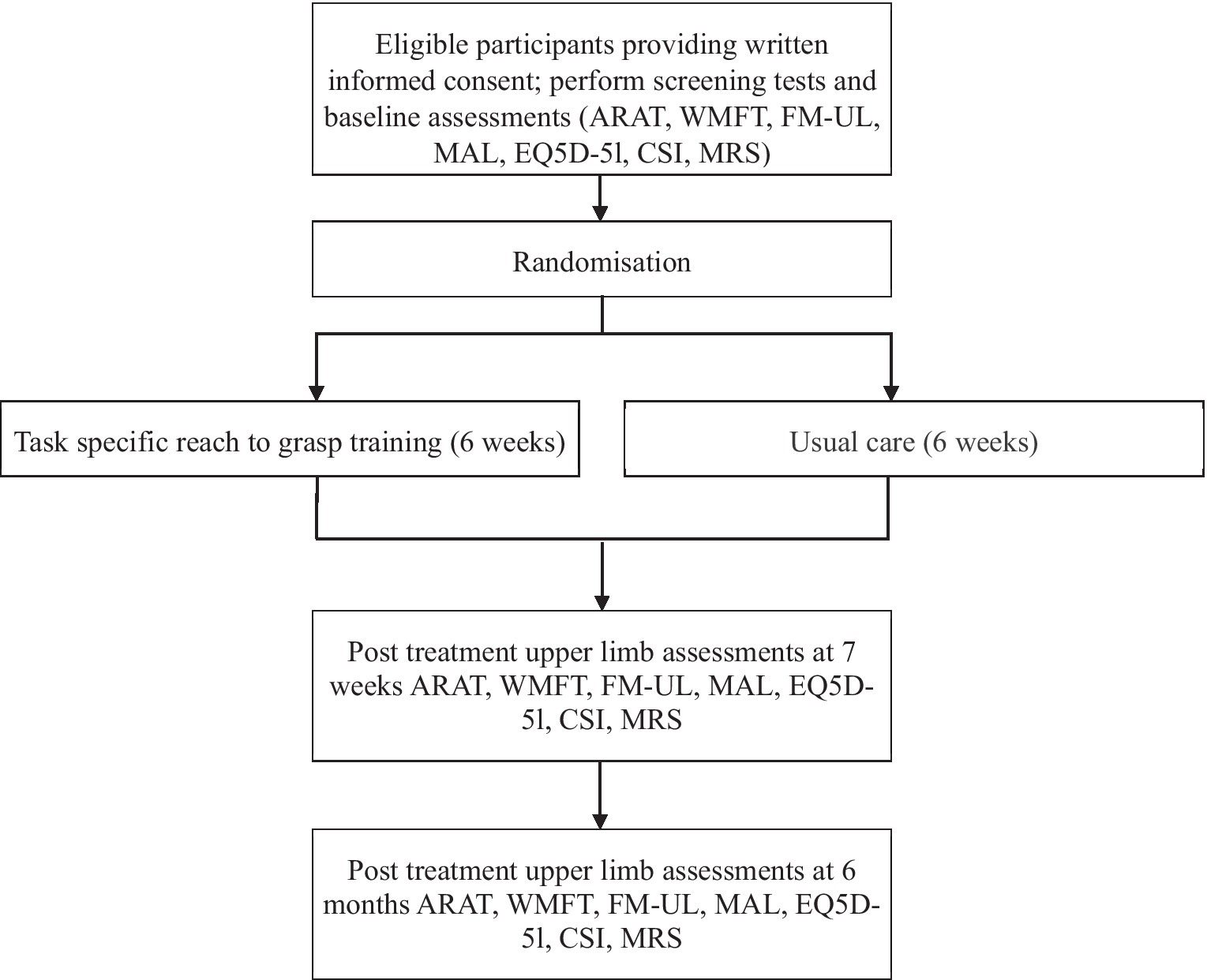
No comments:
Post a Comment