https://www.frontiersin.org/articles/10.3389/fpsyg.2018.00854/full?utm_source=F-AAE&utm_medium=EMLF&utm_campaign=MRK_662896_69_Psycho_20180605_arts_A
- 1Faculdade de Psicologia e de Ciências da Educação, Universidade de Coimbra, Coimbra, Portugal
- 2Madeira Interactive Technologies Institute, Funchal, Portugal
- 3Faculdade de Ciências Exatas e da Engenharia, Universidade da Madeira, Funchal, Portugal
- 4CMM - Centros Médicos e de Reabilitação, Aveiro, Portugal
Stroke is one of the most common causes of acquired
disability, leaving numerous adults with cognitive and motor
impairments, and affecting patients’ capability to live independently.
Virtual Reality (VR) based methods for stroke rehabilitation have mainly
focused on motor rehabilitation but there is increasing interest toward
the integration of cognitive training for providing more effective
solutions. Here we investigate the feasibility for stroke recovery of a
virtual cognitive-motor task, the Reh@Task, which combines adapted arm
reaching, and attention and memory training. 24 participants in the
chronic stage of stroke, with cognitive and motor deficits, were
allocated to one of two groups (VR, Control). Both groups were enrolled
in conventional occupational therapy, which mostly involves motor
training. Additionally, the VR group underwent training with the
Reh@Task and the control group performed time-matched conventional
occupational therapy. Motor and cognitive competences were assessed at
baseline, end of treatment (1 month) and at a 1-month follow-up through
the Montreal Cognitive Assessment, Single Letter Cancelation, Digit
Cancelation, Bells Test, Fugl-Meyer Assessment Test, Chedoke Arm and
Hand Activity Inventory, Modified Ashworth Scale, and Barthel Index. Our
results show that both groups improved in motor function over time, but
the Reh@Task group displayed significantly higher between-group
outcomes in the arm subpart of the Fugl-Meyer Assessment Test.
Improvements in cognitive function were significant and similar in both
groups. Overall, these results are supportive of the viability of VR
tools that combine motor and cognitive training, such as the Reh@Task. Trial Registration: This trial was not registered because it is a small clinical study that addresses the feasibility of a prototype device.
Introduction
Stroke is one of the most common causes of adult disability and its prevalence is likely to increase with an aging population (WHO, 2015).
It is estimated that 33–42% of stroke survivors require assistance for
daily living activities 3–6 months post-stroke and 36% continue to be
disabled 5 years later (Teasell et al., 2012).
Loss of motor control and muscle strength of the upper extremity are
the most prevalent deficits and are those that have a greater impact on
functional capacity (Saposnik, 2016).
Hence, its recovery is fundamental for minimizing long-term disability
and improving quality of life. In fact, most rehabilitation
interventions focus on facilitating recovery through motor learning
principles (Kleim and Jones, 2008).
However, learning engages also cognitive processes such as attention,
memory and executive functioning, all of which may be affected by stroke
(Cumming et al., 2013).
Still, conventional rehabilitation methodologies are mostly motor
focused, although 70% of patients experience some degree of cognitive
decline (Gottesman and Hillis, 2010), which also affects their capability to live independently (Langhorne et al., 2011).
What Is Missing in Conventional Cognitive and Motor Rehabilitation Methodologies?
Although motor and cognitive neurorehabilitation after
acquired brain injury is strongly based on intensive training and
task-specific learning for promoting neural reorganization and recovery (Alia et al., 2017; Galetto and Sacco, 2017), conventional methodologies still strive to accomplish this goal (Levin et al., 2014).
Paper-and-pencil tasks are widely used in cognitive rehabilitation, and
are assumed to be reliable and with adequate construct validity in the
assessment and rehabilitation of cognitive functions after brain injury (Wilson, 1993).
However, this methodology is not suited to deliver immediate feedback
and reinforcement on progress, which is an important element to increase
the motivation and avoid dropouts (Parsons, 2015).
Additionally, when the dominant arm is affected by hemiparesis,
performing paper-and-pencil tasks may become difficult or impossible.
Regarding the motor domain, the persistent repetition of motor actions
can be demotivating due to its repetitiveness and, because it is
laborious and demanding in terms of human resources, it is not as
intensive as it should be (Langhorne et al., 2009).
In addition, the relationship between cognitive and motor deficits is
increasingly being unveiled and cognitive effort appears to contribute
to motor recovery (Pichierri et al., 2011; Mullick et al., 2015; Verstraeten et al., 2016).
Studies with stroke survivors have shown differential patterns of motor
outcomes depending on the cognitive deficits of patients (Čengić et al., 2011; Påhlman et al., 2011).
Moreover, repeated performance of a movement may not lead to meaningful
improvement unless the task is performed within the functional demands
of a relevant environment (Levin et al., 2014).
In fact, the practice of manipulations that require more cognitive
effort were already predicted to be more effective for motor learning
compared to those that require less cognitive effort (Hochstenbach et al., 1998).
In this endeavor, it is important to investigate the learning potential
of patients with post-stroke cognitive and motor impairments by
developing new therapeutic strategies that merge cognitive and motor
intensive training.
Virtual Reality as a Tool for Combined Cognitive and Motor Rehabilitation
Virtual reality (VR) can nowadays be seen as a valuable
approach in stroke rehabilitation, particularly in the motor domain
where studies showed benefits at the level of upper limb function and
ADL (Laver et al., 2017).
This is potentially related to the fact that VR allows creating
conditions to optimize motor learning by promoting meaningful and
iterative practice, together with the delivery of immediate feedback (Levin et al., 2014).
Although less explored, VR also provides the opportunity to integrate
the practice of cognitive and/or motor activities in more ecologically
valid contexts (Rand et al., 2009; Faria et al., 2016a; Adams et al., 2018).
In such scenarios, motor training could be combined with the execution
of cognitive rehabilitation tasks consisting of activities for improving
cognitive domains such as attention, memory, or executive functions.
Moreover, limitations in cognitive function have been shown to have an
effect on VR performance (Kizony et al., 2004),
and thus VR systems should be designed to address different cognitive
profiles. Although the evidence is still modest, some studies with VR
for simultaneous motor and cognitive rehabilitation have shown the
potential of such strategy (Rand et al., 2009; Kim et al., 2011; Lee et al., 2015; Cameirão et al., 2017).
Hence, we argue that novel VR tools should focus on integrative
cognitive and motor rehabilitation based on tasks that pose both
cognitive and motor demands. Assuming the interdependence between the
recovery processes, we may provide a more effective rehabilitation tool.
Here we present the results of a feasibility study with
the Reh@Task, a multi-purpose desktop based virtual scenario that
combines arm reaching and cognitive training through virtual adaptations
for the training of memory and attention of traditional
paper-and-pencil tasks.
Previous Work With the Reh@Task
The Reh@Task is a multi-purpose VR scenario for upper
limb reaching and cognitive training that has been deployed in different
configurations and with different rehabilitation paradigms. It allows
the customization of stimuli, training task and training progression. In
its first version, it originated as an adaptation in VR of the Toulouse
Piéron (TP) cancelation task for the training of attention (Faria et al., 2014).
The prototype was our first attempt to combine motor and cognitive
training. It was primarily an attention only task that consisted on
selecting target elements from a pool of distractors through arm
reaching. This concept was tested in a 1-month intervention case study
with three stroke survivors that presented both motor and cognitive
deficits. Results indicated improvements both at motor and cognitive
levels, suggesting the feasibility of the proposed approach (Faria et al., 2014).
Following those results, the Reh@Task prototype was proposed with
stimuli customization – to encompass varying cancelation tests with
different stimuli – and the incorporation of a memory variant of the
cancelation task for the training of memory, always relying on upper
limb reaching movements. Thus, this new prototype enables the
simultaneous training of upper limb reaching movements, memory, and
attention. One of the advantages of a system such as the Reh@Task is
that it can be easily customized to test different research hypotheses
on the impact of such technology on stroke survivors with different
profiles. In a previous controlled impact study, the Reh@Task was used
to evaluate if cognitive tasks supported by personalized stimuli with
positively valence could lead to improved motor and/or cognitive
outcomes in an understudied population in comparison with standard
rehabilitation. This was done through stimulus selection from
emotionally tagged pictures and through content personalization to
patients’ preferences, including music, in a group of sub-acute stroke
survivors with mild cognitive impairment (MCI) (Cameirão et al., 2017).
Results showed that the Reh@Task was as effective as standard
rehabilitation, although motor and cognitive improvements were poor in
both groups. This suggested that patients with MCI have a poorer
recovery prognostic, specifically when presenting simultaneous motor and
cognitive deficits. In fact, there is evidence that cognitive deficits
interfere with motor recovery (Mullick et al., 2015), and that patients with MCI might have more difficulties in dual-tasking (Schaefer and Schumacher, 2010).
In the present study, the Reh@Task was used with stimuli
different to those used in the above mentioned studies, focusing on
neutral stimuli that do not have an emotional charge and are
traditionally used in standard rehabilitation (symbols, numbers, and
letters), with a difficulty progression based on computational models of
how stimuli properties affect task difficulty (Faria and Bermúdez i Badia, 2015).
Further, in this case our population is chronic. Hence, this study
presents a novel cognitive training, task progression, tested on a
different patient population, and compares the impact of such approach
to time matched conventional rehabilitation activities. We hypothesize
that rehabilitation with the Reh@Task will result in improved motor and
cognitive outcomes when compared to patients in the standard
rehabilitation condition.
Materials and Methods
Experimental Setup and Reh@Task
The setup consists on a PC (OS: Windows 7, CPU: Intel
core 2 duo E8235 at 2.80 GHz, RAM: 4 Gb, Graphics: ATI mobility Radeon
HD 2600 XT), a PlayStation Eye camera (Sony Computer Entertainment Inc.,
Tokyo, Japan) and a customized handle with a tracking pattern. The user
works on a tabletop, facing a LCD monitor (24″) and moves the handle on
the surface of the table with his/her paretic arm (Figure 1A). 2D upper limb reaching movements are captured through a camera-based Augmented Reality (AR) pattern tracking software (AnTS)1 (Mathews et al., 2007).
For adapting the task to individual users, the VR scenario has a
built-in calibration function that normalizes the motor effort required
in the task to the skillset of the user. The movements of the user are
then mapped onto the movements of a virtual arm on the VR environment.
FIGURE 1
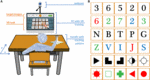 FIGURE 1. Experimental setup and VR task. (A)
The user works on a tabletop and arm movements are captured by
augmented reality pattern tracking. These movements are mapped onto the
movements of a virtual arm on the screen for the execution of the
cancelation task. (B) The target stimuli can be letters, numbers,
and symbols in black or different colors. The target stimuli in this
picture are ordered by increasing complexity.
FIGURE 1. Experimental setup and VR task. (A)
The user works on a tabletop and arm movements are captured by
augmented reality pattern tracking. These movements are mapped onto the
movements of a virtual arm on the screen for the execution of the
cancelation task. (B) The target stimuli can be letters, numbers,
and symbols in black or different colors. The target stimuli in this
picture are ordered by increasing complexity.
The Reh@Task is based on
traditional cancelation tests for the training of attention, and has
been extended to incorporate numbers, letters and symbols, and the
training of memory, and progressive difficulty adjustment according to
the evolution of the patient (Figure 1B).
The task consists on finding target elements within a pool of
distractors. In the memory variant, the targets need to be memorized
first and are hidden during target selection. The VR cancelation task
has incremental difficulty and is adjusted to the individual performance
of each user. There is a total of 120 difficulty levels that were
defined through a participatory design study, where the input of 20
health professionals was operationalized in quantitative guidelines (Faria and Bermúdez i Badia, 2015).
The progression of difficulty is made through the manipulation of the
number of targets and distractors, the type of stimulus, the time
available to solve the task, the time for selection and, in the memory
variant of the task, the amount of time for memorizing the target. These
parameters are all operationalized in a way that increases the
difficulty of the task incrementally (see Faria et al., 2016b)
for further details on the difficulty adjustment algorithm). In
summary, for higher difficulty levels, more target and distractor
elements appear, less time is available for completing the task and
memorizing the target images, and action selection is quicker. When a
patient does not solve a specific level in the established timing, more
time is given for that level. This additional time can be incremented up
to three times. If the user fails three times in a row, he/she goes
back to the previous level. If the user succeeds, the level must then be
successfully performed within the original established time.
Finally, a rule was defined to select the starting level in each training session according to:
where StartLevel and EndLevel
denote the starting and finishing levels, respectively, and t indicates
the session number. For instance, if the level achieved by a participant
in the first session was 28, the second session would start in level 14
(28/2). If in the second session level 44 would be reached, the third
session would start in level 29 [14 + (44 - 14)/2], and so on for the
following levels.
Participants
The sample was a convenience sample with a final size of
24 participants recruited at two outpatient rehabilitation units of CMM –
Centros Médicos e Reabilitação (Murtosa and Aveiro, Portugal) between
June of 2015 and April of 2017. The inclusion criteria were the
following: chronic stroke (>6 months); undergoing occupational
therapy rehabilitation at CMM; motor impairment of the upper extremity
with sufficient observable movement to perform the virtual task,
corresponding to a minimum score of 28 in the Motricity Index (MI) (Demeurisse et al., 1980)
for elbow flexion and shoulder abduction combined; cognitive deficit
but with enough capacity to understand the task and follow instructions,
as assessed by the therapists; and able to read and write. Exclusion
criteria included: history of premorbid deficits; unilateral spatial
neglect assessed through paper-and-pencil cancelation tests; severe
depressive symptomatology with a score above 20 points in the Geriatric
Depression Scale (GDS) (Yesavage et al., 1983);
and vision disorders that could interfere with the execution of the
task. Thirty-two stroke survivors were included and randomized for
participation in this study. Minor deviations from inclusion/exclusion
criteria were permitted for two participants, and did not affect the
participants’ health, wellbeing, and rights (1 participant was 5 months
post-stroke; 1 participant had a GSD score of 22). 25 participants
completed the protocol, 1 dropped out, and 6 did not fulfill the
experimental protocol. One participant was not included in the analysis
because this participant was later confirmed to be in the acute stage of
stroke (Figure 2). Hence, 24 participants (12 in VR group, 12 in Control group) were included in the analysis (Table 1).
There were no significant differences between groups in demographics,
except for age, the control group was significantly older (Mann–Whitney,
U = 31.0, p = 0.017). This study was carried out in
accordance with established ethical guidelines and was approved by the
board of CMM – Centros Médicos e Reabilitação. All participants gave
written informed consent in accordance with the Declaration of Helsinki.
FIGURE 2
TABLE 1
Experimental Protocol
This study followed a between-subjects design. After
recruitment and baseline assessment, the participants were randomly
assigned to one of two groups (VR or Control) by a researcher not
involved in data collection, using the Research Randomizer, a free
web-based service that offers instant random sampling and random
assignment (Research Randomizer2).
Participants in the VR group underwent 12 sessions of 45 min with the
Reh@Task, three times a week, for 1 month. Before the first session,
participants went through an average of three short training trials with
the Reh@Task with TP abstract stimuli. The training was intended to
provide a clear understanding of the VR task, as well as to become used
to the natural user interface (AnTS). After assuring that the patient
understood the task and interface instructions, the intervention started
with the attention variant of the task, then switched to memory, and so
on intermittently. The control group intervention was time-matched and
included twelve sessions of 45 min of standard occupational therapy,
spatial and time orientation activities, and writing training. Both
interventions were in addition to conventional occupational therapy that
typically entails 2–3 weekly sessions of 45–60 min and includes upper
limb motricity training, practice of fine motor skills, cognitive-motor
training, dexterity training, ADL, normalization of muscle tone, balance
training and communication training. Participants underwent motor and
cognitive assessment through a number of standardized clinical scales,
at baseline, end of treatment and 1-month follow-up.
Cognitive, Motor, and Functional Assessment
Cognitive and motor scales that are widely applied
clinically and in research were used to determine impairment severity
and to measure cognitive and motor recovery. The assessor was not blind
for the type of intervention. The cognitive profiling was made through
the Montreal Cognitive Assessment (MoCA) (Freitas et al., 2011),
which provides sub-scores for the following domains: Executive
Functions, Naming, Memory, Attention, Language, Abstraction, and
Orientation. The attention task-related capabilities were assessed with
the Single Letter Cancelation (SLC) (Diller et al., 1974), the Digit Cancelation (DC) (Mohs et al., 1997) and the Bells Test (BT) (Gauthier et al., 1989). Motor deficits were assessed through the upper extremities part of the Fugl-Meyer Assessment Test (FM-UE) (Fugl-Meyer et al., 1975)
for motor and joint functioning of the paretic upper extremity. Of the
total score of 66, we also analyzed separately proximal (shoulder,
elbow, forearm, coordination, 42/66) and distal (wrist, hand, 24/66)
function. For functionality of the paretic upper extremity, the Chedoke
Arm and Hand Activity Inventory (Barreca et al., 2004)
(CAHAI) was used. MI was used to assess muscle power of the paretic
upper extremity. Spasticity was assessed through the Modified Ashworth
Scale (MAS) (Bohannon and Smith, 1987). Finally, the Barthel Index (BI) (Mahoney and Barthel, 1965) was used to assess independence in activities of daily living (ADLs).
Data Analysis
The normality of distributions was assessed using the
Kolmogorov–Smirnov test for normality. Because most distributions
deviated from normality, non-parametric statistical tests were used.
Hence, central tendency and dispersion measures of the variables are
presented as median and interquartile range (IQR), respectively. For
improvements in clinical scores, we show the mean and standard deviation
(SD) for an easier comparison with the literature. Differences between
groups in demographic and clinical data at baseline were assessed using a
Mann–Whitney U test in interval and ordinal variables, and a Pearson’s chi-square (χ2)
test in nominal variables. A per-protocol analysis was used. For
within-group changes over time across the three evaluation moments
(baseline, end of treatment, and follow-up), a Friedman test for related
samples was used and reported as χ2 (degrees of freedom).
The Wilcoxon’s T matched pairs signed ranks (one-tailed because we
predicted improvement over time in both groups) was used for further
related pairwise comparisons with respect to baseline. No correction was
applied to account for the number of pairwise comparisons, as
non-parametric tests are already considered conservative. To compare
groups at the end of treatment and follow-up, for each group we computed
the improvement with respect to baseline. We used a one-tailed
Mann–Whiney U test to test the hypothesis that improvements in the VR group were superior against the control group.
The Reh@Task software logged data on patient task
performance (errors, number of targets and distractors, type of stimuli,
time to completion) as well as the movement traces of the paretic arm,
smoothed using a Gaussian window of 1 second. Performance improvements
over time in the VR group were assessed by comparing the performances of
each patient at the first and last training sessions. The error rates
were computed as a percentage for each type of stimulus during the 12
training sessions. Movement smoothness was computed from the movement
traces by counting the number of movement sequences, defined as
trajectory segments in-between null acceleration points. To assess
improvements in range of movement (ROM) over time, changes in the
tracked position of the hand were assumed in the x- and y-axis
of the tabletop surface, and the average improvements of the last three
sessions were compared against the average of the 3 first sessions. All
comparisons were performed using the two-tailed Wilcoxon’s T matched pairs signed ranks test.
Effect sizes (r) are reported on the pairwise comparisons and are computed as Z/√N (Rosenthal, 1991).
The criteria for interpretation of the effect is 0.1 = small, 0.3 =
medium, and 0.5 = large. For all statistical tests, a significance level
of 5% (α = 0.05) was set. Data were analyzed using Matlab (MathWorks
Inc., Natick, MA, United States) and IBM SPSS Statistics for Windows,
Version 22.0 (Armonk, NY, United States: IBM Corp).
Results
How Effective Is Cognitive Training With Reh@Task as Compared to Conventional Rehabilitation?
The baseline MoCA total scores were balanced between groups (U = 60.5, p = 0.503, r = 0.18), and so were the scores in MoCA subdomains (data not shown). Also balanced were the number of errors in SLC (U = 64.5, p = 0.659, r = 0.09), DC (U = 57.5, p = 0383, r = 0.19), and BT (U = 58.5, p = 0.431, r = 0.16).
The analysis of the scores over time for each group,
considering the three evaluation moments (baseline, end of treatment,
and follow-up), showed a significant impact on MoCA total score and some
of its subdomains in both groups (Table 2). Specifically, the VR group displayed a significant effect in MoCA-Total [χ2(2) = 8.3, p = 0.016], MoCA-Recall [χ2(2) = 6.2, p = 0.046], and MoCA-Orientation [χ2(2) = 8.4, p = 0.015]. The control group showed a significant effect in MoCA-Total [χ2(2) = 9.1, p = 0.010], MoCA-Language [χ2(2) = 6.1, p = 0.047], and MoCA-Recall [χ2(2) = 6.1, p
= 0.048]. Further pairwise comparisons with respect to baseline
indicated that for the MoCA total score, both groups showed a
significant improvement at end of treatment [VR: T = 12.5, Z = 1.83, p = 0.034, r = 0.37; Control: T = 3.0, Z = 2.68, p = 0.003, r = 0.55], and follow-up [VR: T = 2.0, Z = 2.62, p = 0.004, r = 0.53; Control: T = 2.0, Z = 2.77, p = 0.003, r
= 0.56]. Mean improvements in MoCA total score at end of treatment were
2.6 ± 4.3 in VR against 3.1 ± 2.8 in Control, and for follow-up 3.4 ±
3.5 in VR against 3.0 ± 3.0 in Control. For MoCA subdomains with
significant effects over time, improvements were also significant at end
of treatment and follow-up for both groups. For the cancelation tests,
the VR group showed a significant effect over time for BT [χ2(2) = 6.6, p
= 0.037] only. Pairwise comparisons with respect to baseline revealed
that this effect comes from a significant improvement at follow-up (T = 2.5, Z = 2.40, p = 0.016, r = 0.49), but not at the end of treatment. The control group showed a significant effect over time for the DC [χ2(2) = 11.3, p = 0.004] and BT [χ2(2) = 10.5, p
= 0.005], with significant improvements at end of treatment and
follow-up. No significant differences were found in the between-groups
analysis, when comparing the significant improvements in the VR group
with those of the control group at end of treatment and follow-up.
TABLE 2
 TABLE 2. Scores in cognitive assessment at baseline, end of treatment and follow-up for VR and control conditions.
TABLE 2. Scores in cognitive assessment at baseline, end of treatment and follow-up for VR and control conditions.How Effective Is Motor Training With Reh@Task as Compared to Conventional Rehabilitation?
On the scores in motor assessment scales at baseline, the groups were balanced in the CAHAI (U = 43.0, p = 0.093), BI (U = 56.5, p = 0.360), and MAS (U = 54.0, p = 0.281). However, the groups were not balanced in FM-UE (U = 28.5, p = 0.010) and MI (U = 33.0, p = 0.024), with the control group having significantly higher scores in these two scales.
The analysis of the scores over time for each group,
considering the three evaluation moments, showed for both groups a
significant impact on FM-UE [VR: χ2(2) = 12.1, p = 0.002; Control: χ2(2) = 11.1, p = 0.004], CAHAI [VR: χ2(2) = 7.5, p = 0.023; Control: χ2(2) = 11.3, p = 0.004], and MI [VR: χ2(2) = 12.0, p = 0.002; Control: χ2(2) = 11.3, p = 0.004] (Table 3). On the FM-UE arm and hand subparts, both groups showed significant improvements over time for the hand domain [VR: χ2(2) = 8.4, p = 0.015; Control: χ2(2) = 7.7, p = 0.021], but only the VR group improved significantly in the arm part [VR: χ2(2) = 11.1, p = 0.004; Control: χ2(2) = 4.7, p = 0.097]. The control group showed an additional significant effect in MAS [χ2(2) = 7.6, p
= 0.022], indicating a decrease in spasticity. There was no significant
effect over time for BI. Further pairwise comparisons with respect to
baseline indicated that for the VR group improvements were significant
at end of treatment and follow-up in FM-UE [End: T = 0.0, Z = 2.20, p = 0.014, r = 0.45; Follow-up: T = 0.0, Z = 2.37, p = 0.009, r = 0.48], FM-Arm [End: T = 0.0, Z = 2.21, p = 0.013, r = 0.45; Follow-up: T = 0.0, Z = 2.20, p = 0.014, r = 0.45], FM-Hand/wrist [End: T = 0.0, Z = 1.83, p = 0.034, r = 0.37; Follow-up: T = 0.0, Z = 2.03, p = 0.021, r = 0.41], CAHAI [End: T = 0.0, Z = 1.86, p = 0.031, r = 0.40; Follow-up: T = 0.0, Z = 1.89, p = 0.029, r = 0.39], and MI [End: T = 7.5, Z = 1.78, p = 0.037, r = 0.36; Follow-up: T = 1.0, Z = 2.85, p = 0.002, r = 0.58]. For FM-Arm, the improvement compared to the control group was significantly higher (U = 45.0, p = 0.031, r = 0.38) at end of treatment and marginally significant at follow-up (U = 48.0, p = 0.055, r = 0.33). The control group showed significant improvements at end of treatment and follow-up in FM-UE [End: T = 0.0, Z = 2.03, p = 0.021, r = 0.41; Follow-up: T = 0.0, Z = 2.38, p = 0.008, r = 0.49], FM-Hand/wrist [End: T = 1.0, Z = 1.75, p = 0.040, r = 0.36; Follow-up: T = 0.0, Z = 2.21, p = 0.013, r = 0.45], CAHAI [End: T = 0.0, Z = 2.23, p = 0.013, r = 0.45; Follow-up: T = 0.0, Z = 2.21, p = 0.013, r = 0.45], and MI [End: T = 0.0, Z = 2.04, p = 0.020, r = 0.42; Follow-up: T = 0.0, Z = 2.38 p = 0.009, r = 0.48]. For the MAS, the improvements were only significant at follow-up [End: T = 0.0, Z = 1.41, p = 0.078, r = 0.29; Follow-up: T = 0.0, Z = 2.24, p = 0.012, r
= 0.46], corresponding to a median decrease of one grade in this
spasticity scale, specifically from 1+ to 1. Besides the significant
difference in FM-Arm at end of treatment, no other significant
differences were found in the between-groups analysis at end of
treatment and follow-up.
TABLE 3
 TABLE 3. Scores in motor assessment at baseline, end of treatment and follow-up for VR and control conditions.
TABLE 3. Scores in motor assessment at baseline, end of treatment and follow-up for VR and control conditions.
The mean improvements with
respect to baseline at end of treatment and follow-up in the measures
where a significant within-group effect over time was observed are
presented in Table 4.
For the VR and control groups, the observed average improvement in
FM-UE was 4.6 ± 6.2 and 2.1 ± 3.6, respectively. This improvement in the
VR group mainly comes from the FM-Arm subpart and strongly contrast
with what was measured in the control group at end of treatment (3.7 ±
5.1 in VR against 0.8 ± 2.0 in Control, p = 0.031) and follow-up (4.0 ± 5.5 in VR against 0.9 ± 2.1 in Control, p
= 0.055). The average improvements in the FM-Hand/wrist subpart,
although being significant with respect to baseline, were modest for
both groups at end of treatment (0.8 ± 1.4 in VR against 1.3 ± 2.3 in
Control) and follow-up (0.9 ± 1.4 in VR against 1.8 ± 2.1 in Control).
Also modest were the improvements in the CAHAI for both groups at end of
treatment (0.8 ± 1.5 in VR against 2.7 ± 3.1 in Control) and follow-up
(1.1 ± 1.8 in VR against 4.3 ± 4.9 in Control). These values are
considerably below of what is considered a Minimal Detectable Change
(MDC), which should be above 6.3 (Barreca et al., 2005).
For the MI, the average improvements were higher in VR when compared to
control at end of treatment (4.8 ± 8.3 in VR against 3.9 ± 5.4 in
Control) and follow-up (9.1 ± 8.7 in VR against 5.3 ± 5.4 in Control),
although not being significantly different.
TABLE 4
Outcomes in Reh@Task Measures
Task Performance Measures
The Reh@task data allowed us to quantify the evolution
of patients in the VR group over time in between assessment points.
Several variables are considered for this analysis: difficulty level
achieved during each training session, type of task (memory/attention),
and type of stimulus.
When looking at changes over time, we observe that
patients improve over time in both task types but display a deceleration
as levels of higher difficulty are achieved (Figure 3).
Patients achieve in average higher difficulty levels in the attention
task, display a steeper slope, and exhibit a constant variability over
time. In contrast, improvements in the memory task are slower, reaching
lower difficulty levels and with increasing variability over time,
indicating an uneven increased difficulty of this task in patients when
compared to attention. Data show significant improvements in task
performance between the first and last sessions [Attention: Z = 2.99, p = 0.003, r = 0.61; Memory: Z = 3.07, p = 0.002, r = 0.63] (Figure 4). There were comparable performances in the first session for both attention (M = 35.5 ± 11.3) and memory tasks (M
= 30.3 ± 8.2), but the difference is statistically significant in the
last training session [Attention: 51.3 ± 8.0, Memory: 43.5 ± 11.9, Z = 2.64, p = 0.008, r = 0.54].
FIGURE 3
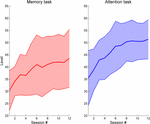 FIGURE 3. Task performance evolution over time in
the Reh@Task. Data show the maximum difficulty level achieved per
training session for the memory and attention tasks.
FIGURE 3. Task performance evolution over time in
the Reh@Task. Data show the maximum difficulty level achieved per
training session for the memory and attention tasks.
FIGURE 4
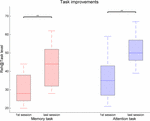 FIGURE 4. Task performance changes between the first
and last training sessions for the memory and attention tasks in the
Reh@Task. The whiskers indicate the most extreme data points that are
not considered outliers. ∗∗ indicates p < 0.01.
FIGURE 4. Task performance changes between the first
and last training sessions for the memory and attention tasks in the
Reh@Task. The whiskers indicate the most extreme data points that are
not considered outliers. ∗∗ indicates p < 0.01.
If task performance is analyzed by type of stimulus, distinct performances can be seen (Figure 5).
An increasing average number of errors is observed for Numbers (6.5%),
Letters (10.4%), and Symbols (17.5%), and the difference is significant
when comparing symbols and numbers (Z = 2.12, p = 0.034, r
= 0.43), showing a continuum of difficulty that is consistent with the
level of abstraction of each category. In addition, all categories show a
significantly increased error rate when comparing the black stimuli
with their colored counterpart [Numbers: Z = 3.06, p = 0.002, r = 0.62; Letters: Z = 2.98, p = 0.003, r = 0.61; Symbols: Z = 2.43, p = 0.015, r
= 0.50]. Interestingly, error rates are similar for colored numbers
(25.50%) and for colored symbols (25.48%) despite numbers being easier
than symbols when uncoupled with colors. Surprisingly, error rates are
significantly lower for colored letters than for colored numbers
[Colored Letters: 17.81%, Colored Numbers: 25.50%, Z = 2.12, p = 0.034, r = 0.43].
FIGURE 5
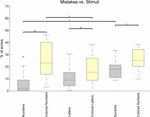 FIGURE 5. Percentage of task mistakes depending on
the category of stimulus being presented in the Reh@Task. The whiskers
indicate the most extreme data points that are not considered outliers.
Outliers are represented as +. ∗ or ∗∗ indicates p < 0.05 or p < 0.01, respectively.
FIGURE 5. Percentage of task mistakes depending on
the category of stimulus being presented in the Reh@Task. The whiskers
indicate the most extreme data points that are not considered outliers.
Outliers are represented as +. ∗ or ∗∗ indicates p < 0.05 or p < 0.01, respectively.Motor Performance Measures
The analysis arm movement trajectories provide
information on both ROM and movement smoothness. The movement smoothness
metric assumes that the movement trajectories that are built of less
movement segments, that is, with less accelerations and decelerations,
are indicative of a more controlled and smooth movement. A comparison of
movement smoothness between the first and the last training sessions
revealed a very significant decrease in the number of movement segments,
indicating longer and smoother trajectories (Z = 2.93, p = 0.003, r = 0.60) (Figure 6).
Finally, an analysis of the changes in ROM as assessed by the system’s
calibration at the beginning of each session revealed significant
improvements in the x (30.1% of improvement, Z = 2.67, p = 0.008, r = 0.54) component of the movement, but not on the y (Figure 7).
FIGURE 6
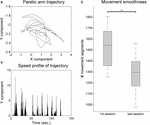 FIGURE 6. Movement smoothness analysis for the VR group. (A) Example 2-min sample of movement trajectory of one patient. (B) Computed speed profile of the sample in (A). Movement sequence segments are identified in-between null acceleration points. (C)
Movement smoothness changes between the first and last training
sessions. The whiskers indicate the most extreme data points that are
not considered outliers. ∗∗ indicates p < 0.01.
FIGURE 6. Movement smoothness analysis for the VR group. (A) Example 2-min sample of movement trajectory of one patient. (B) Computed speed profile of the sample in (A). Movement sequence segments are identified in-between null acceleration points. (C)
Movement smoothness changes between the first and last training
sessions. The whiskers indicate the most extreme data points that are
not considered outliers. ∗∗ indicates p < 0.01.
FIGURE 7
 FIGURE 7. Changes over time of the x and y
component of the Range of movement as assessed by the Reh@Task
calibration. The whiskers indicate the most extreme data points that are
not considered outliers. Outliers are represented as +. ∗∗ indicates p < 0.01.
FIGURE 7. Changes over time of the x and y
component of the Range of movement as assessed by the Reh@Task
calibration. The whiskers indicate the most extreme data points that are
not considered outliers. Outliers are represented as +. ∗∗ indicates p < 0.01.Discussion
We presented a randomized controlled study with a VR
cognitive and motor training task, the Reh@Task, consisting on a 1-month
intervention with 24 chronic stroke survivors. We compared time-matched
training with Reh@Task to standard occupational rehabilitation. During
the intervention, all patients underwent conventional occupational
therapy; only the VR group had specific training with the Reh@Task. The
goal of this study was to investigate the benefits for stroke recovery
of an integrative VR approach that combines cognitive and motor
training. The main hypothesis behind this approach is that when
approaching both motor and cognitive components, the context and
situatedness of training impact its ecological validity. For this
reason, both motor and cognitive challenges are personalized to each
patient and presented as a single motor-cognitive VR task.
Our data show that both groups improved significantly in
the motor domain in the FM-UE, CAHAI, and MI. However, in the total
FM-UE the improvements in the VR group (4.6–4.9) were on average twice
of those for the control group (2.1–2.7). This improvement in VR is
superior to the ones observed in previous studies with similar VR
paradigms in a chronic population (Cameirão et al., 2012; Maier et al., 2017). A more intensive (20 sessions in 1 month) motor-only intervention resulted on FM-UE improvements of about three points (Cameirão et al., 2012).
A combined cognitive-motor approach, where the cognitive domain did not
follow an automated adjustment approach but was more intensive (5
weekly sessions of 30 min during 6 weeks), led only to average
improvements of less than 2 points in FM-UE (Maier et al., 2017).
An analysis of our results in the FM components indicates that the
improvement in the FM-Arm is significantly higher in comparison to
control. Although both groups address proximal movements, this could be
attributed to the nature of the VR task, which focuses on reaching
movements. This is in line with other cognitive-motor studies with
chronic stroke survivors where the training of hand motor competences in
VR resulted in gains on manual abilities (Broeren et al., 2008).
Nevertheless, our VR task does not address distal movements and
comparable FM-Hand/Wrist improvements with the control group are
achieved. These improvements in clinical scales are consistent with the
Reh@Task data, that showed significant gains in ROM and movement
smoothness. Concerning spasticity as measured by the MAS, we observed a
significant reduction of one grade (from 1+ to 1) for the control but
not the VR group. This is most likely related to the fact that the
control group underwent more time of conventional occupational therapy,
which includes normalization of muscle tone. Nevertheless, it has been
argued that the 1+ and 1 grades do not have enough granularity do
discriminate changes in spasticity (Pandyan et al., 1999).
Motor improvements did not generalize into clinically
meaningful improvements in ADLs as measured by the BI and CAHAI.
Considering that our sample is chronic and presents a very high BI and a
low CAHAI at baseline, this indicates that these patients have high
levels of independence despite their deficits. This suggests that
effective strategies have been learned prior to the study that do not
involve the paretic arm, leading to learned non-use, commonly observed
in chronic populations (Wolf et al., 1989). If this is the case, an effective VR training should also incorporate strategies to address learned non-use (Ballester et al., 2016).
This hypothesis is supported by previous results of an intervention
with a modified version of the Reh@Task in a subacute population, in
which improvements in CAHAI were larger, reaching meaningful values (Cameirão et al., 2017).
This is also consistent with data from another integrative
cognitive-motor VR study with patients in the 1st month post-stroke,
where a mean improvement in BI of ∼20 points was registered (Kim et al., 2011), what strongly contrasts with the average 5 points improvement that we measured in our study with a chronic population.
The impact of both VR and control interventions in
cognitive function was significant (3/30 in MoCA) but not different
between groups. Still, our results strongly contrast with those obtained
using a similar motor and cognitive training paradigm with chronic
stroke where improvements in cognitive function where not significant
after 6 weeks of training (Maier et al., 2017),
despite being a more intensive training with five sessions a week. Both
groups in our study showed improvements in total MoCA and recall, which
suggests that both interventions had an impact in terms of general
cognitive functioning and memory. VR showed an additional improvement in
orientation, and the control group in language. The lack of
improvements in other sub-domains could be explained by the fact that
although MoCA has high sensitivity to detect post-stroke cognitive
impairment (Godefroy et al., 2011),
it is a screening tool and might have not fully detected the specific
cognitive impact of this intervention. Both groups improved in attention
as assessed by the cancelation tests. Hence, the VR group had
improvements consistent with the dimensions trained in the Reh@Task, and
consistent with the Reh@Task performance data. The performance data
during VR training show significant improvements over time in both
memory and attention training. The lower performance in the memory tasks
is also consistent with the lower recall scores of MoCA at baseline.
The analysis of task performance depending on the stimulus used supports
the importance of the modeling effort of our personalization algorithm,
which automatically adjusts the task configuration (including stimulus
type, number of targets, and distractors) to provide an appropriate
challenge to the patient.
A prototype version of the Reh@Task, combining attention
and arm reaching only, was previously tested with three chronic stroke
survivors in a less intensive intervention (Faria et al., 2014).
In that pilot study, two patients showed improvements in motor and
cognitive function, and in ADLs, indicating the potential of an approach
that integrates motor and cognitive training. Later, a different
customization of the Reh@Task was used in a controlled study with
subacute stroke survivors (Cameirão et al., 2017).
The intervention was time-matched to the one being presented here and
contrasting results were obtained. In that case, the Reh@Task was
configured to also train attention, memory and arm reaching, but
pictures of positive valence were used instead. In terms of mean
improvements, in the here presented study we observed higher
improvements in total FM-UE (4.6–4.9 against 0.3–3.0) and MoCA (2.6–3.4
against -0.9–1.7), and lower improvements in CAHAI (0.8–1.1 against
6.6–11.1). These results are interesting because it would be expected to
observe a higher impact of training in the subacute population, but
this was not the case. The subacute population improved poorly in both
motor and cognitive domains. A factor that could contribute to this
result is the fact that the subacute population had higher cognitive
deficits at baseline (median 20.0 against 22.5), and it has been
suggested that cognitive functioning is associated with upper limb motor
recovery (Mullick et al., 2015).
Additionally, the subacute population had on average higher depressive
symptomology (15.1 against 11.2) and less years of schooling (4.6
against 6.0). Both these factors have been associated with poorer
cognitive performance (Zahodne et al., 2015; MacIntosh et al., 2017).
However, the subacute population did better in the performance of ADLs
as measured by the CAHAI. As previously mentioned these differences
could be related to learned non-use that is often observed in chronic
stroke patients, that limits the impact of actual rehabilitation gains (Wolf et al., 1989). This highlights the importance of an early use of rehabilitation strategies that prevent learned non-use.
We believe that the presented results are supportive of
the viability of low-cost rehabilitation solutions that combine motor
and cognitive training, such as the Reh@Task. These solutions show
potential to be effective tools to address cognitive training in an
integrative manner and can be easily deployed at home or at the clinic.
Our data supports a larger impact in motor function than in cognitive
function when compared to control. One possible reason could be the
limited range of cognitive tasks implemented in Reh@Task that do not
encompass all domains needed to be addressed in a comprehensive
rehabilitation program. A second reason could be the limited ecological
validity of the training tasks. Despite being integrative
motor-cognitive tasks, these are still far from actual motor-cognitive
tasks performed in ADLs. Previous work using VR cognitive training of
ADLs in simulated environments like a virtual mall or a virtual city
showed translation of competences to real world ADLs (Rand et al., 2009) and improved outcomes when compared to standard cognitive rehabilitation (Faria et al., 2016a).
The relevance of such approaches can also be seen in a recent study
with chronic stroke survivors that used a VR scenario for motor training
based on the execution of virtual ADLs (Adams et al., 2018).
After 8 weeks of treatment, a group of 15 patients showed a mean
improvement of ∼6 points in FM-UE, which is superior to what we have
observed in our study.
Although further research in this area is essential, this
work presents a valuable step toward designing more effective
rehabilitation technologies that combine motor and cognitive training
relying on VR. In fact, the recent Cochrane review on the effect of VR
in stroke rehabilitation reports that there are not enough studies to
assess the impact of VR in cognitive function (Laver et al., 2017).
Hence, we believe that our contribution is relevant to the field.
Nevertheless, this study has some limitations that should be considered.
First, due to sequential admittance into the study, we used a
completely randomized design, resulting in a heterogeneity of groups in
age and FM baseline measures. The fact that groups differ in FM may also
imply different recovery profiles. Second, although the use of standard
of care as control is necessary, this control did not train the exact
same competences as the Reh@Task. Third, the use of screening
instruments for the assessment of the improvements in cognitive function
in this context may lack the sensitivity to capture small improvements
in the different domains addressed.
 Ana L. Faria
Ana L. Faria Mónica S. Cameirão
Mónica S. Cameirão Joana F. Couras
Joana F. Couras Joana R. O. Aguiar
Joana R. O. Aguiar Gabriel M. Costa
Gabriel M. Costa Sergi Bermúdez i Badia
Sergi Bermúdez i Badia


No comments:
Post a Comment