http://www.ptproductsonline.com/2016/06/technological-advances-rehabilitation-gait-balance-stroke/
By Mithu Lijo, Msc PT, NCS, CBIS, MSCS, and Jamie Bolt, PT, DPT, NCS
Stroke ranks as the sixth highest cause of burden of disease worldwide in terms of disability.1 About 60% of people who have had a stroke have difficulties with walking, and 30% of people affected with stroke do not regain complete motor recovery after rehabilitation.2 One of the primary concerns for individuals who experience stroke is the ability to regain walking, with respect to safety, speed, balance, and postural control. Consequently, gait retraining is a key focal area during rehabilitation following stroke. Even after intensive rehabilitation focusing on gait and balance training, stroke survivors still present with significant spatiotemporal asymmetry and balance deficits that interfere with their independence and quality of life.
Rehabilitation in the acute phase is very important in the recovery of function. The 2016 AHA adult stroke guidelines recommend inpatient rehabilitation to improve quality of life and return to previous level of function.2 From the time of admission, patients are engaged in an intensive rehabilitation program to improve their mobility and self care skills in different environments and life situations. Advances in technology enable patients who are affected by a wide range of physical impairments to begin participating in rehabilitative activities without becoming apprehensive about falls and risk of injury to both patients and staff.1 Neurologic physical therapists use various technologies to address gait and balance impairments in stroke patients to enable them to return to a productive life. This article examines the use of technological advances to address three specific impairments in gait following stroke: speed, spatiotemporal asymmetry, and dynamic balance.
Speed
Walking speed is directly related to functional independence and community mobility after stroke. On average, two out of three stroke survivors are unable to walk at a speed that enables them to function independently in the community. The literature suggests an average walking velocity of2 .1 m/s to 1.5 m/s is considered fast enough to be functional as a pedestrian in different environmental and social contexts (eg, crossing a street safely).3 At a walking speed of more than 0.8 m per second, full mobility in the community is likely; at a walking speed of less than 0.4 m per second, mobility is limited to the home; and at speeds of 0.4 to 0.8 m per second, mobility is limited to short walks in the community.3 Walking on a treadmill, with or without body weight supported via a harness connected to an overhead support system, is a method of treating walking impairments post stroke that is becoming increasingly popular.4One highly recognized piece of equipment is the LiteGait (Mobility Research, Tempe, Ariz) with body weight support treadmill training, to enable nonambulatory or limited ambulatory hemiparetic stroke patients to practice coordinated stepping:
• during progressively increased demands for postural control;
• with control over gait speed;
• with potential to stimulate normal walking pattern; and
• with reduced oxygen demand.
The use of body weight support (BWS) allows the walking process to be comfortable for the patient. Such support provides the ability to focus on stepping with the paretic leg with assistance from the therapist. It also reduces the need for intensive support from therapists during gait training, which often leads to fatigue and risk of injury. It in fact results in lower intensity of therapy for the patient. Use of a treadmill permits a greater number of steps to be performed within a training session, thereby increasing the amount of task-specific practice completed. Hesse, 2003, reported that people after stroke can perform up to 1,000 steps in a 20-minute treadmill training session, compared with only 50 to 100 steps during a 30-minute session of conventional physical therapy.5
Cochrane systematic review concluded that patients with stroke, who received electro-mechanics assisted gait training in combination with physical therapy, were more likely to achieve independent walking than those who do not.2 The review also found that the individuals most likely to benefit from this therapy appear to be those who are within the first 3 months after stroke as well as those who are unable to walk. Treadmill training (with or without BWS) at higher speeds (2.0 mph) is more effective at improving walking after stroke than training at slower speeds.6 In addition, the task-specific nature of gait training on the treadmill requires the patient to engage in walking practice with high repetition. Such training results in long-term changes in functional ability. During BWS treadmill training, prescription of specific parameters such as percent of body weight support, speed of the treadmill, support stiffness, and handrail hold can affect treatment outcomes in stroke patients.7
Ankle dorsiflexor weakness affects 20% to 30% of patients undergoing rehabilitation and has been identified as a predictor of decreased walking speed and endurance.8 Electrical stimulation orthotic substitute devices, such as the Bioness L300 Foot Drop System (Bioness, Valencia, Calif) and WalkAide (Innovative Neurotronics, Reno, Nev), have been shown to improve gait speed in patients who require dorsiflexion assist during acute, subacute, and chronic phases of stroke recovery.9 Increased patient satisfaction, confidence in walking, and compliance have also been reported with the use of electrical stimulation devices compared to AFOs in poststroke patients.8 These devices have the advantage of monitoring a patient’s compliance with a walking program at home and facilitating correct kinematics without manual assistance both on a treadmill with or without BWS and overground walking.
Spatiotemporal Symmetry
Gait asymmetry is a particularly important problem for stroke survivors due to(i) reduced walking speed;
(ii) deficits in propulsive force production;
(iii) reductions in dynamic standing balance; and
(iv) musculoskeletal imbalances, which can lead to
• pain;
• reductions in general activity levels; and
• reductions in loading, which can affect bone density and reduced efficiency of walking.
Rehabilitation targeting gait symmetry is an important consideration for treatment, during both the acute and the chronic stages of stroke rehabilitation. Studies have shown that cortical and subcortical strokes causing a range of motor and sensory deficits did not impair a person’s ability to make immediate reactions or slower adaptations during split-belt locomotion.10 Instead, it could temporarily store a new interlimb relationship, producing a more normal gait pattern.10
One method of improving gait symmetry in stroke patients is through the use of split-belt treadmill training. It is an adaptation paradigm where one leg is driven to move faster than the other. In this method, the patients learn a new spatial and temporal coordination pattern between limbs when the belts are driven at different speeds. A critical component of split-belt treadmill training is the error augmentation that provides the nervous system with a cue to correct the asymmetry. Studies have shown that 4 weeks of error-augmenting patient specific split-belt training can lead to more symmetric over-ground walking patterns.11 Therapists should consider each individual patient’s baseline asymmetry before commencing use of split-belt treadmill, since the same split-belt perturbation can result in different after-effects.12 The recommended speed for split belt treadmill training is at a rate of 2:1.12 The patient’s baseline asymmetry decides which leg will be placed on the slow moving belt. Table 1 explains the recommended paradigms for correcting temporospatial gait asymmetry.12
Technologies such as the GAITRite (CIR Systems Inc, Franklin, NJ) and Zeno Walkway (ProtoKinetics, Havertown, Pa) provide quantitative measures of spatio-temporal parameters of gait such as cadence, step length, double support as a percentage of gait cycle, and velocity. It enables the therapist to devise appropriate treatment plans to correct specific gait asymmetries, monitor effectiveness of intervention over time, and select appropriate assistive devices. The GAITRite system and the Zeno Walkway have been used with balance and gait outcome measures such as TUG, 6MWT, and 10MWT to monitor the changes in spatio-temporal gait variables with different functional tasks and its significance on activities of daily living.
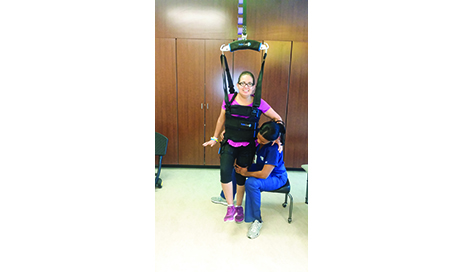
Body
weight support allows the walking process to be comfortable for the
patient. It also reduces the need for intensive support from therapists
during gait training, which often leads to fatigue and risk of injury.
Proactive and Reactive Balance
Falls are one of the most common medical complications after stroke, and are major safety concern during rehabilitation. The incidence ranges from 7% in the first week after stroke to 25%-36% from the first month to sixth months poststroke.2 Between 6 and 12 months, the incidence probability increases to 70%.2 This has a significant impact on a stroke survivor’s quality of life and cost of healthcare. The reduced confidence in one’s ability to balance and move around in an upright position leads to fear of falls with limited independence, functional mobility, and participation in ADLs.Retraining of dynamic standing balance is an integrative component of stroke rehabilitation. Dynamic BWS systems such as the SafeGait 360° Balance and Mobility Trainer (Gorbel Inc-Medical Division, Fishers, NY), Vector (Bioness, Valencia, Calif) and ZeroG (Aretech, Ashburn, Va) allows therapists to work on proactive and reactive balance in standing without risk of falls. It simultaneously creates confidence in patients to practice functional activities. In addition, the different functional tasks incorporated in the SafeGait system enable the patient and therapist to keep track of the number of repetitions and efficiency of tasks during each session. SafeGait also enables the patient and therapist to work on pre-gait activities, overground walking, managing steps, curbs, quick turns and reaching in multiple directions simulating normal environment.
Biodex Balance System SD (Biodex Medical Systems Inc, Shirley, NY) is another technological advance available in the rehabilitation of balance in poststroke patients. It offers postural stability training, weight shift training in multiple directions, fine and random movement training, and fall risk screening. It allows the clinician to study the postural control recovery of stroke patients and provides audio-visual and proprioceptive feedback to patients to improve weight bearing asymmetry and strategies to work on improving postural control and balance.
Toward Increased Intensity, Efficiency, and Quality in Gait Rehabilitation
Gait training incorporating electro-mechanical devices assists therapists and patients to work on improving the quality and intensity of gait training without risking safety and fatigue from early on. In the current healthcare environment, patients are faced with reduced inpatient hospital days and outpatient therapy visits. There is a high expectation from insurance companies to improve functional mobility. The variety of electromechanical devices available, such as SafeGait, Split Belt Treadmill, LiteGait, Vector, ZeroG, Bioness L300 Foot Drop System, WalkAide, etc, enables therapists to address specific impairments in gait and balance, thereby improving quality of life, reducing risk of falls and additional healthcare costs. PTPMithu Lijo, Msc PT, NCS, CBIS, MSCS, is a physical therapist at TIRR Memorial Hermann Hospital, The Woodlands, Texas. She has more than a decade of international experience across Asia, Europe, and the United States. She has a postgraduate degree in Neurological Physical Therapy from Sheffield Hallam University, United Kingdom (UK). She also holds Basic Bobath certification from the UK. She has specialized experience in the rehabilitation of stroke, brain injury, spinal cord injury, and various neurodegenerative disorders.
Jamie Bolt, PT, DPT, NCS, has practiced in multiple specialty areas, including acute care, adult inpatient rehab, women’s health, cancer rehabilitation, lymphedema therapy, and vestibular rehabilitation. Jamie received a certification in vestibular rehabilitation from Emory University School of Medicine in 2013. Jamie has worked for TIRR Memorial Hermann in The Woodlands since opening in 2013 and is now the clinical coordinator of Physical Therapy services. Jamie also is a LSVT-certified clinician and enjoys working with patients with degenerative diseases. For more information, contact PTPEditor@allied360.com.
References
1. Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2013 Jul 25;(7):CD006185.
2. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169.
3. Duncan PW, Sullivan KW, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026-36.
4. Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2014 Jan 23;(1):CD002840.
5. Hesse S. Treadmill training with partial body weight support after stroke: A review. NeuroRehabilitation. 2008;23(1):55-65.
6. Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87(12):1580–1602.
7. Sheffler LR, Chae J. Technological advances in interventions to enhance post-stroke gait. Phys Med Rehabil Clin N Am. 2013 May;24(2):305-323.
8. Bosch PR, Harris JE, Wing K, American Congress of Rehabilitation Medicine (ACRM) Stroke Movement Interventions Subcommittee. Review of therapeutic electrical stimulation for dorsiflexion assist and orthotic substitution. From the American Congress of Rehabilitation Medicine Stroke Movement Interventions Subcommittee. Arch Phys Med Rehabil. 2014;95(2):390-396.
9. Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87(6):853-859.
10. Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007 July;130(Pt 7):1861-1872.
11. Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009 Sep;23(7):735-744.
12. Malone LA, Bastian AJ. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil Neural Repair. 2014 Mar-Apr;28(3):230-240.
No comments:
Post a Comment