So what the fuck do we do with this information? With nothing to suggest this is totally fucking useless.
But do you want gout because of this?
Gout may lessen Alzheimer risk
Or take this for gout?
Could Old Gout Drug Offer New CV Benefits?
Uric Acid Contributes to Obesity-Paradox of the Outcome of Ischemic Stroke
- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
Background: The mechanism of obesity
paradox in stroke is not clear. This study aimed to investigate whether
uric acid (UA) contributes to obesity-stroke outcome paradox.
Material and Methods: The study cohort
consisted of 1,984 IS patients recruited in the ACROSS-China study.
Serum UA and BMI were measured at admission. Low and high BMI groups
were defined by the threshold of 24, and low and high UA by the age- and
sex-specific median. Poor outcomes were defined as modified Rankin
scale score ≥3 in 1 year after onset.
Results: UA was significantly and
positively correlated with BMI. Lower levels of UA and BMI were
significantly associated with higher risk of poor outcomes. Incidence of
the poor outcome was 34.5, 29.4, 27.7, and 23.5% in the BMI/UA groups
of low/low, high/low, low/high and high/high, respectively, with p = 0.001 for trend. The association between low UA and poor outcome was significant in lower BMI groups (odds ratio = 1.36, p = 0.006 in quartile 1 and 1.28, p = 0.021 in quartile 2), but the odds ratios were not significant in the BMI quartile 3 and 4 groups, with p = 0.038 for trend. The adverse effect of lower UA was significant in males, but not in females, with p = 0.006 for sex difference.
Conclusions: These findings suggest that
low UA and low BMI have a joint effect on poor outcomes in IS patients.
Across BMI categories, uric acid is differentially associated with
functional outcome after stroke. This effect of low UA in the low BMI
groups may be one of the mechanisms underlying the obesity-stroke
paradox of the outcome in IS patients.
Introduction
Uric acid is the final oxidation product of purine metabolism and a water-soluble antioxidant and radical scavenger in humans (1, 2).
Despite the antioxidant properties of uric acid, epidemiologic studies
have shown that hyperuricemia is related to an increased risk of
cardiovascular events, stroke, and their risk factors (3–6).
On the other hand, growing evidence has been emerging that elevated
uric acid concentration is associated with better functional outcomes of
ischemic stroke (IS) (7–11).
Moreover, the exogenous administration of uric acid exerts robust
neuroprotective effect on the outcome of IS patients in clinical trials (12, 13).
Obesity, a metabolic disorder, has a wide range of negative effects on health consequences (14). There is consistent evidence that obesity is an established independent risk factor for incidence of IS (15). However, many studies, but not all, suggest a better prognosis in overweight and obese patients after IS (16–19).
The improved survival and functional outcomes, and stroke recurrence in
overweight and obese IS patients are termed as “obesity-stroke paradox”
in the literature (18, 19).
Although the obesity-stroke paradox related factors and metabolic
pathways have been documented in previous studies, the underpinning
mechanisms are still not fully understood (20, 21).
Oxidative stress is a major contributor to brain damage in patients with IS (22). Uric acid, a powerful antioxidant, is highly and positively correlated with body weight (6).
To date, the joint effect of uric acid and obesity on the IS outcomes
has not been reported in previous studies. The working hypothesis of
this study is that uric acid concentration contributes to the
obesity-stroke outcome paradox in patients with IS. The current study
aims to investigate the synergistic effect of uric acid and body mass
index (BMI) on clinical functional outcomes after IS in a 1-year
follow-up study cohort.
Materials and Methods
Participants and Procedures
The Abnormal gluCose Regulation in patients with acute
strOke acroSS China (ACROSS-China) is a hospital-based multicenter
prospective cohort study. The ACROSS-China study focuses on the
prevalence and distribution of abnormal glucose regulation in patients
with acute stroke and the impact of abnormal glucose regulation on the
outcome of stroke patients (11). A total of 3,450 patients were recruited from 34 hospitals across China in 2008–2009, including patients with IS (n = 2,639), intracerebral hemorrhage (n = 649) and subarachnoid hemorrhage (n = 162). Acute stroke was diagnosed according to World Health Organization diagnostic criteria (23).
The patients were followed for 1 year, and information on clinical
outcomes was obtained by personal interview. Among the 2,639 patients
with IS, 1,984 patients who had serum uric acid data available and
completed the follow-up interview survey for the outcome formed the
current study cohort. This study is a retrospective analysis of the
ACROSS-China cohort. Characteristics of the patients who were included
and excluded (n = 655) were presented in Supplemental Table 1.
The study was approved by the Central Institutional Review Board at
Beijing Tiantan Hospital. Written informed consent was obtained from all
the patients or their representatives.
Replicate measurements of height and weight were made,
and the mean values were used for analysis. BMI (weight in kilograms
divided by height in meters squared) was used to measure adiposity. Low
and high BMI was defined by the threshold of 24 kg/m2, based on
recommendations of the Working Group on Obesity in China (24).
Serum uric acid was measured at admission by the urine enzyme endpoint
method. Low and high uric acid was defined as being below and above the
age- and sex-specific medians. Fast glucose was measured by using an
enzymatic method. Estimated glomerular filtration rate (eGFR) was
calculated using the Chronic Kidney Disease Epidemiology Collaboration
creatinine equation with adjusted coefficient of 1.1 for the Asian
population. Information, including age, sex, smoking, alcohol drinking,
disease history, time from symptom onset to admission and medications
was obtained by means of a nurse-administered standardized questionnaire
at the time of admission. Current smoking and drinking were defined as
smoking at least one cigarette per day and consuming alcohol every day,
respectively.
At 1 year after stroke onset, the prognosis of all the
patients were assessed through a centralized telephone follow-up. The
primary clinical outcome of this study was defined as death or major
disability at the end of 1-year follow-up period. Modified Rankin scale
(mRS) score ranging from 3 to 6 was defined as major disability or
death. A score of 0 indicated no symptoms, a score of 5 indicated severe
disability, i.e., bedridden, incontinent, or requiring constant nursing
care and attention, and a score of 6 indicated death (25). Death events were confirmed through death certificates from the local citizen registry or by the attended hospital.
Statistical Analyses
Mean ± standard deviation (SD) or median (interquartile
range [IQR]) was used for describing continuous variables, and
frequencies and percentages for categorical variables. Differences in
distribution of dichotomous variables between subgroups were tested by
Chi-square tests. Uric acid and BMI were analyzed as both continuous and
dichotomous variables. The relationship of BMI and uric acid with the
outcome was examined in multivariable logistic regression models.
Covariates included in the model for adjustment were age, sex, time from
symptom onset to admission, smoking, alcohol drinking, National
Institutes of Health stroke scale (NIHSS), stroke subtype, fast glucose,
eGFR, blood pressure, and lipid-lowering and antihypertensive
medications. Odds ratio (OR) and 95% confidence interval (CI) were
estimated in logistic regression models. The significance of differences
in ORs of uric acid between BMI quartile groups was tested in logistic
regression interaction models. All statistical analyses were performed
using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
Table 1
summarizes characteristics of study variables by sex groups and in the
total sample. Males were younger and had greater prevalence of smokers
and alcohol drinkers, lower prevalence of anti-hypertensive treatment
and lower glucose, NIHSS than females. Males had lower BMI but higher UA
and eGFR than females. The difference in incidence rates of poor
outcome (mRS ≥ 3) between sex groups was significant, with males having a
lower rate than females.
TABLE 1
Table 2
shows the relationship of uric acid, BMI, age, sex, and diabetes.
Female sex was significantly associated with lower uric acid, but higher
BMI. Older age was significantly associated with higher uric acid and
lower BMI. IS patient with diabetes had lower uric acid, but higher BMI.
Of note, BMI was significantly and positively correlated with uric
acid.
TABLE 2
Figure 1
presents incidence rates of poor outcome (mRS ≥ 3) in 1-year follow-up
by uric acid and BMI groups. Low and high BMI was defined by the
threshold of 24, and low and high uric acid by the median. As shown in Panel A, the lower uric acid group showed a higher incidence rate of poor outcome than the higher uric acid group (31.6 vs. 25.0%, p = 0.001); the lower BMI group showed a higher incidence rate of poor outcome than the higher BMI group (31.4 vs. 26.3%, p = 0.013). Panel B
showed incidence rates of poor outcome by groups of combined BMI/uric
acid. Incidence of the poor outcome was 34.5, 29.4, 27.7, and 23.5% in
the BMI/uric acid groups of low/low, high/low, low/high and high/high,
respectively, with p = 0.001 for trend.
FIGURE 1
 Figure 1. (A,B) Incidence of poor outcome (mRS ≥ 3)
by BMI and uric acid groups. Low and high BMI was defined by the
threshold of 24, and low and high UA by the sex-specific medians. BMI,
body mass index; mRS, modified Rankin Scale; UA, uric acid.
Figure 1. (A,B) Incidence of poor outcome (mRS ≥ 3)
by BMI and uric acid groups. Low and high BMI was defined by the
threshold of 24, and low and high UA by the sex-specific medians. BMI,
body mass index; mRS, modified Rankin Scale; UA, uric acid.
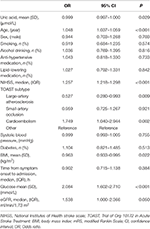
Table 3
shows the association of BMI and uric acid with outcome, adjusting for
covariates. Older age was significantly associated with higher risk of
worse outcome. Patients with higher NIHSS were 1.26 times more likely to
have worse outcome. Higher blood glucose was significantly associated
with higher risk of poor outcome. Higher levels of BMI and uric acid had
significantly protective effects on the outcome. The interactions of
uric acid were significant with sex (p = 0.001) and BMI (p = 0.045), but not significant with age (p = 0.313), systolic blood pressure (p = 0.487) and diabetes (p = 0.974).
TABLE 3
Figure 2
presents odds ratio (OR) and 95% confidence interval (CI) of lower uric
acid for poor outcome (mRS ≥ 3) by BMI quartile (panel A) and sex
(panel B) groups, with quartile 4 of uric acid (the highest) as
reference. The association between lower uric acid and poor outcome was
significant in lower BMI groups (OR = 1.36, p = 0.006 in quartile 1; OR = 1.28, p
= 0.021 in quartile 2), but the ORs were not significant in BMI
quartile 3 and 4 groups. The interaction between uric acid and BMI was
significant, with p = 0.038 for the decreasing trend in ORs
across increasing BMI quartiles. The association between low uric acid
and poor outcome was significant in males (OR = 1.31, p < 0.001), but not significant in females (OR = 0.96, p = 0.618), with p = 0.006 for sex difference.
FIGURE 2
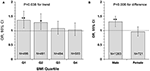 Figure 2. Odds ratio and 95% confidence interval of lower uric acid for poor outcome (mRS ≥ 3) by BMI quartile (A) and sex (B) groups, with quartile 4 of uric acid (the highest) as reference. *P < 0.05 and **P < 0.01 for OR >1. BMI, body mass index; CI, confidence interval; mRS, modified Rankin Scale; OR, Odds ratio.
Figure 2. Odds ratio and 95% confidence interval of lower uric acid for poor outcome (mRS ≥ 3) by BMI quartile (A) and sex (B) groups, with quartile 4 of uric acid (the highest) as reference. *P < 0.05 and **P < 0.01 for OR >1. BMI, body mass index; CI, confidence interval; mRS, modified Rankin Scale; OR, Odds ratio.
Figure 3
presents OR and 95% CI of lower uric acid for poor outcome by sex/BMI
groups, with quartile 4 of uric acid (the highest) as reference. The
adverse effect of lower uric acid measured as OR was significant in
males, but not in females, with p = 0.004 for the trend. Of note, the difference in ORs between low and high BMI groups was not significant in males (p = 0.512 for interaction) and females (p = 0.335 for interaction).
FIGURE 3
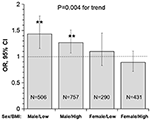 Figure 3. Odds ratio and 95% confidence interval of
lower uric acid for poor outcome (mRS ≥ 3) with quartile 4 of uric acid
(the highest) as reference by sex/BMI groups. Low and high BMI was
defined by the threshold of 24; **P < 0.01 for OR >1. BMI, body mass index; CI, confidence interval; mRS, modified Rankin scale; OR, Odds ratio.
Figure 3. Odds ratio and 95% confidence interval of
lower uric acid for poor outcome (mRS ≥ 3) with quartile 4 of uric acid
(the highest) as reference by sex/BMI groups. Low and high BMI was
defined by the threshold of 24; **P < 0.01 for OR >1. BMI, body mass index; CI, confidence interval; mRS, modified Rankin scale; OR, Odds ratio.Discussion
Obesity is an escalating pandemic worldwide, representing an emerging threat to public health (26, 27).
The obesity association with cardiovascular and cerebrovascular
diseases is well-demonstrated. Studies have concluded that obesity is
strongly associated with increased stroke risk in both men and women (28, 29).
Although the adverse health consequences of obesity in the general
population have been well-documented, strong evidence suggests that
obesity is associated with improved survival rate and functional
outcomes and lower stroke recurrence in patients with IS, indicating the
existence of an “obesity-stroke paradox” (16–19).
However, some studies supported that no obesity paradox exists in
patients with IS after adjustment for initial neurological severity (30–32).
The existence of obesity paradox is still controversial. In the current
study, the higher BMI (≥24) group had a significantly lower incidence
of poor functional outcome (mRS ≥ 3) than the lower BMI (<24) group
(26.3 vs. 31.4%, p = 0.013) in a 1-year follow-up of IS patients;
BMI was significantly and negatively associated with the risk of poor
functional outcome in multivariable logistic regression models,
adjusting for covariates. Despite strong observational data indicating a
survival benefit of obese patients after stroke, methodological
concerns exist (16, 33),
and the biologic mechanisms contributing to pathways in metabolic
imbalance underlying the obesity-stroke paradox are largely unknown (20, 21).
Data from most observational studies, systematic
reviews, and meta-analyses support the concept that elevated uric acid
concentration is associated with better functional outcomes of IS (7–11).
Patients with acute IS have a 12% increase in the odds of good clinical
outcome for each milligram per deciliter increase of serum uric acid (34).
Moreover, a meta-analysis of 10 studies found that high serum uric acid
level was associated better outcome after acute IS as compared to low
serum uric acid level (9).
Furthermore, one prospective study suggested that the increased uric
acid levels are associated with better outcome in IS patients treated
with reperfusion therapy (35).
Similarly, another study demonstrated that early elevation of uric acid
during or shortly after IS onset presented significant protection
against neurological deficit in acute IS patients treated with
recombinant tissue plasminogen activator (rt-PA) (36).
The ACROSS-China study reported that lower serum uric acid levels
strongly predicted short-term poor functional outcome in acute stroke
with normoglycaemia (11).
In addition, evidence for the beneficial effect of exogenous
administration of uric acid on the outcome of IS patients in clinical
trials has been emerging in recent years (12, 13).
We found in this study that lower uric acid levels were significantly
associated with poor functional outcome of IS patients, and this effect
was exacerbated by lower BMI. The joint effect of uric acid and obesity
status on the IS outcome has not been reported in previous studies. The
detrimental impact of lower uric acid observed in the present study may
be one of metabolic mechanisms of the obesity-stroke outcome paradox.
Obesity, a metabolic disorder, is well-known to
correlate with uric acid. The Bogalusa Herat Study has shown that BMI
levels were significantly, positively associated with uric acid in both
children and adults (6).
It is also noted in current study cohort that lower BMI was
significantly correlated with decreased levels of uric acid in IS
patients. Incidence of poor outcome (mRS ≥ 3) was the highest in the low
BMI-low uric acid group and was the lowest in the high BMI-high uric
acid group, with p for trend = 0.001 (Figure 1B).
Uric acid concentrations decrease significantly over time in stroke
patients, and the plasma antioxidant capacity has been inversely
correlated with the volume of cerebral infarction and the severity of
neurological impairment (37).
IS patients with lower BMI tended to have lower uric acid which was
associated with decreased antioxidant capacity. The highest OR of low
uric acid for poor outcome in IS in the lowest BMI quartile group (Figure 2A)
indicated a synergistic effect of lower BMI and lower uric acid on the
poor outcome. The findings on the interaction effect between BMI and
uric acid on the IS outcome suggested that lower BMI levels might be
associated with poor outcome in IS, at least in part, through lower uric
acid concentrations. Studies with specifically sophisticated design in
other populations are needed to confirm our findings. Further
investigations should be performed in acute IS patient treated with
rt-PA to further explore whether same results can be observed.
Another interesting finding in this study is the
sex-specific association between uric acid and the outcome in IS.
Despite the significant difference in the incidence of poor outcome
(males < females), the association between lower uric acid and the
poor outcome was significant in male patients, but not in female
patients. The male-low BMI group showed the highest OR (1.43), and the
female-high BMI group showed the lowest OR (0.89), with p for trend =
0.004 (Figure 3B).
In a prospective study of the China Antihypertensive Trial in Acute
Ischemic Stroke, the association between serum uric acid and primary
outcomes of IS measured as death and major disability (mRS ≥ 3) at 3
months was modified by sex (P for interaction n = 0.007) in 3284
acute IS patients. Elevated serum uric acid was significantly and
positively associated with the primary outcome in men, but not in women (38).
In contrast, the administration of uric acid reduced infarct growth in
patients with acute IS treated with alteplase in women, but not in men
in a clinical trial in Spain (39).
As we know, uric acid level is different between sex, and men usually
have a higher uric acid level than women. Women with higher uric acid
levels were significantly associated with the development of
hypertension and metabolic syndrome, however, it was not found in men.
We hypothesized that exogenous administration of uric acid may have a
greater benefit for male patients with low uric acid level in acute
ischemic stroke with or without reperfusion therapies. To date, data on
the sex-specific association between uric acid and outcomes in IS are
still limited. Prospective double blind randomized controlled trials in
this regard are required to investigate the effect of uric acid on the
outcomes in male and female IS patients.
There are several limitations of our study include:
First, the result of this study could not generalized to western
population, because there is much difference between western population
and Chinese population in the distribution of BMI. Second, our study has
significant missing data about serum uric acid and the included
population has higher proportion of diabetes, lipid-lowering medication,
and higher BMI level compared with excluded population. Thus,
popularizing the results to general population is limited. Further
investigations in other larger populations are needed to confirm the
findings. Third, the telephone follow-up for outcome assessment is
sub-optimal. Fourth, this study is a hospital-based cohort, which may
lead to selection bias. A large population-based study is needed to
confirm our findings. Fifth, the cohort was recruited between 2008 and
2009, therefore, it was considered not contemporary. Sixth, the lack of
information on use of acute reperfusion therapies in this study may
hampered further investigation on the relationship between uric acid and
obesity-stroke paradox in patients treated with reperfusion therapies.
Conclusions
In summary, this observational prospective study
highlighted that across BMI categories, uric acid was differentially
associated with functional outcome after stroke. Of note, a synergistic
effect of uric acid and BMI was found that the association between low
levels of uric acid and the poor clinical functional outcome was
strengthened by lower BMI in IS patients. The detrimental effect of low
uric acid on the outcome was observed to be significant in male, but not
in female IS patients. The findings of the present study have
implications in understanding the metabolic mechanisms of the
obesity-stroke outcome paradox, help identify high-risk patients of IS
and provide additional evidence for the administration of uric acid in
clinical trial studies. Given the practical implications that could be
derived in the field of prevention of poor outcome in IS, larger studies
would be required to confirm these encouraging results.
 Hefei Tang1,2,3,4†,
Hefei Tang1,2,3,4†,  Jinglin Mo
Jinglin Mo Zimo Chen
Zimo Chen Yongjun Wang
Yongjun Wang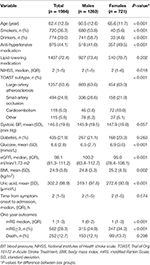

No comments:
Post a Comment