Remote ischemic postconditioning (RIPostC) is the application of a transient and brief ischemic stimulus to a distant site from the organ or texture that is afterward exposed to injury ischemia[8] and has been found to reduce IRI in various animal models.
This might be
leg wraps (9 posts to May 2013)
leg compressions (19 posts to September 2015)
If so, then your hospital and doctors are extremely incompetent for not having protocols on this in place.
The effect of repeated remote ischemic postconditioning after an ischemic stroke (REPOST): a randomized controlled trial
Trials volume 20, Article number: 167 (2019)
Abstract
Background
Remote ischemic postconditioning (rIPostC) refers to the observation that repeated, short periods of ischemia protect remote areas against tissue damage during and after prolonged ischemia. Based on previous observations of a potential neuroprotective effect of rIPostC, the aim of this study is to evaluate whether repeated rIPostC after an ischemic stroke can reduce infarct size, which could be translated to an improvement in clinical outcomes.
Methods/design
We will enroll 200 ischemic stroke patients to daily rIPostC or sham conditioning during hospitalization into a randomized single-blind placebo-controlled trial. The intervention consists of twice daily exposure to four cycles of 5-min cuff inflation around the upper arm to > 20 mmHg above systolic blood pressure (i.e., rIPostC) or 50 mmHg (i.e., control), followed by 5 minutes of deflation. The primary outcome is infarct size, measured using an MRI diffusion-weighted image at the end of hospitalization. Secondary outcomes include the Modified Rankin Scale, National Institutes of Health Stroke Scale, quality of life, and cardiovascular and cerebrovascular morbidity and mortality. To explore possible underlying mechanisms of rIPostC, venous blood will be sampled to assess biomarkers of inflammation and vascular health.
Discussion
Previous studies in animals and humans, using a single bout of remote ischemic conditioning, report a potential effect of rIPostC in attenuating neural damage. Although repeated rIPostC has been investigated for cardiovascular disease patients and preclinical stroke models, no previous study has explored the potential physiological and clinical effects of repeatedly applying rIPostC during the hospitalization phase after a stroke.
Trial registration
Netherlands Trial Register, NTR6880. Registered on 8 December 2017.
Background
Ischemic stroke is the leading cause of disability in adults worldwide and has the second highest mortality of all cardiovascular diseases [1]. In particular, the intravenous and endovascular treatment of ischemic stroke has markedly improved survival and long-term functional outcomes [2,3,4]. Unfortunately, a substantial number of stroke patients still end up with a physical disability. This may in part be because the therapeutic window to attenuate the detrimental impact of ischemic injury is limited to 6 hours after the stroke. Currently, there are no subsequent treatment options available [5], although some recent studies have had promising results for intra-arterial therapy in specific subgroups of stroke patients [6, 7]. Moreover, acute revascularization might induce ischemia reperfusion injury [8] and glutamatergic excitotoxicity [9], which could lead to additional damage in the ischemic penumbra. This highlights the need for innovative additional treatment options, which preferably would extend beyond the 6 hours post-stroke. Identification of such treatment options may have a significant impact on the burden of stroke for individual patients, their caregivers, health-care professionals, and at the socioeconomic level.
A potential approach may be the application of remote ischemic postconditioning (rIPostC). Remote ischemic conditioning (RIC) consists of several cycles of brief periods (5 min) of limb ischemia followed by reperfusion, which can be applied by inflating a simple blood pressure cuff. This intervention subsequently confers protection against severe ischemia in remote organs in humans [10,11,12]. Due to the unpredictability of stroke, applying RIC before the event is not possible. However, similar protective effects are present when RIC is applied during or even after an ischemic insult [13], which is known as rIPostC. Whilst the majority of studies have focused on the protective effects of ischemic pre- and postconditioning on cardiac tissue [10,11,12, 14, 15], recently, several studies have supported the ability of rIPostC to reduce neural damage after reperfusion [8, 16,17,18,19], validating that rIPostC may have clinical potential for stroke patients, with clinical trials showing promising results [20, 21]. Additionally, Hess et al. postulated that, in addition to the short-lasting benefits of a single bout of conditioning, chronic benefits may be induced with repeated daily conditioning [18]. This has since been supported by preclinical studies in mice and rats [22, 23] and further supported by evidence from clinical trials in humans with favorable clinical outcomes for repeated rIPostC in ischemic stroke patients [24, 25].
We, therefore, hypothesized that rIPostC during the first few days following an ischemic stroke reduces infarct size. Since infarct size is strongly related to functional recovery [26], repeated rIPostC may be a simple and novel strategy to minimize the clinical impact of an ischemic stroke. Importantly, rIPostC is virtually cost-free, non-pharmacological, and non-invasive, and it can easily be added to the current treatment of stroke patients.
Methods/design
Aim
The aim of this trial is to examine the effect of repeated (twice daily) rIPostC during hospitalization on infarct size and clinical outcomes in ischemic stroke patients. In addition, we aim to explore the potential underlying (inflammatory) mechanisms for this effect of repeated rIPostC.
Design
We will perform a randomized single-blind placebo-controlled clinical trial at the Radboud University Medical Centre (Radboudumc) in Nijmegen, the Netherlands. The study has been approved by the relevant ethical committee (CMO Arnhem-Nijmegen, registration number 2017–3711). This protocol has been registered with the Netherlands Trial Register (NTR6880).
Patient population and randomization
Patients with an ischemic stroke who are being admitted to the emergency room of the Radboudumc are eligible for this study. Other inclusion criteria are: older than 18 years and a clinical diagnosis of an ischemic stroke (established by a neurologist, based on the World Health Organization criteria for stroke) [27]. Exclusion criteria include: unstable vital signs, admitted more than 12 hours after onset of symptoms, upper extremity injury, edema contra-indicating rIPostC, bilateral mastectomy, or axillary lymph node dissection. Patients with contra-indications for magnetic resonance imaging (MRI; e.g., those with a pacemaker, vascular clips, cochlear implants, or other implanted metal objects) will also be excluded because of the impossibility of assessing our primary outcome. All patients will provide written informed consent. For those patients who are not mentally capable of signing informed consent in the acute setting, oral assent will be obtained. This will allow the intervention to start while providing an opportunity for informed consent to be given when the patients or their caregivers are capable of making a well-considered decision. Patients who receive a change in diagnosis after inclusion will be excluded from the analysis of our primary outcome (infarct size).
After informed consent or oral assent has been given, randomization will be performed using a predefined table generated by the computer program SealedEnvelope. Patients will be randomized in a 1:1 ratio to rIPostC or sham conditioning by the coordinating investigator. Stratification will be performed for treatment received (thrombectomy, thrombolysis, or no revascularization treatment) to ensure equal allocation of these subgroups to rIPostC and sham conditioning.
Intervention: repeated remote ischemic postconditioning
A manual blood pressure cuff will be placed around the upper arm after the diagnosis of an ischemic stroke. All participants will receive four cycles of 5-min inflation of the blood pressure cuff, followed by 5 minutes of reperfusion. This procedure will be performed twice daily (morning and afternoon) during the hospitalization after the ischemic stroke for a maximum of 4 days. The level of cuff inflation differs between the groups. Cuff inflation in the rIPostC-group is >20 mmHg above systolic blood pressure, mediating full blockage of the arterial inflow. Subjects in the control group receive cuff inflation to 50 mmHg, which will not induce any ischemia. The intervention will be administered by a trained researcher. Blood pressure will be recorded before the intervention.
Primary outcome measure
The primary outcome measure will be the infarct size on day 4 after admission (or at discharge if discharge is before day 4), which will be compared between the intervention and control groups. The infarct size will be evaluated by diffusion-weighted MRI brain imaging using a 1.5-tesla MRI scanner (Siemens® Avanto). The infarct size will be annotated manually and analyzed on a dedicated work station by a trained researcher under the supervision of a neuroradiologist.
Secondary outcome measures
(1) The validated and frequently used Modified Rankin Scale (mRS) [2, 28, 29] will be used to assess clinical outcomes after 12 weeks. To assess clinical outcomes at the end of hospitalization, the validated National Institutes of Health Stroke Scale [30] will be used. Both scores are calculated as standard after a stroke and will be assessed by a clinical physician or nurse from the neurology department.
After 12 months, we will also examine hospitalization, recurrent (cerebro)vascular events, and mortality. These data will be extracted from patient files at the Radboudumc.
(2) To assess quality of life, participants will be asked to fill out two questionnaires after 12 weeks and 1 year. The first questionnaire is the Stroke-Specific Quality of Life Scale (SSQoL), which is reliable and reproducible for self-reported quality of life outcomes in stroke patients [31]. The second questionnaire is the TOPICS-SF (The Older Persons and Informal Caregivers Survey Short Form), which is used for assessing patient-reported outcome measures. The TOPICS-SF has been shown to be a valid and feasible questionnaire for older patients [32, 33].
(3) Venous blood will be sampled during the first presentation at the emergency department and at the end of hospitalization to explore the impact of daily rIPostC on vascular, immune, and anti-inflammatory pathways. Whole blood will be spun down and serum will be stored at −80 °C for future analysis. For further analysis of possible underlying mechanisms, urine will also be sampled and stored at −80 °C at the end of hospitalization.
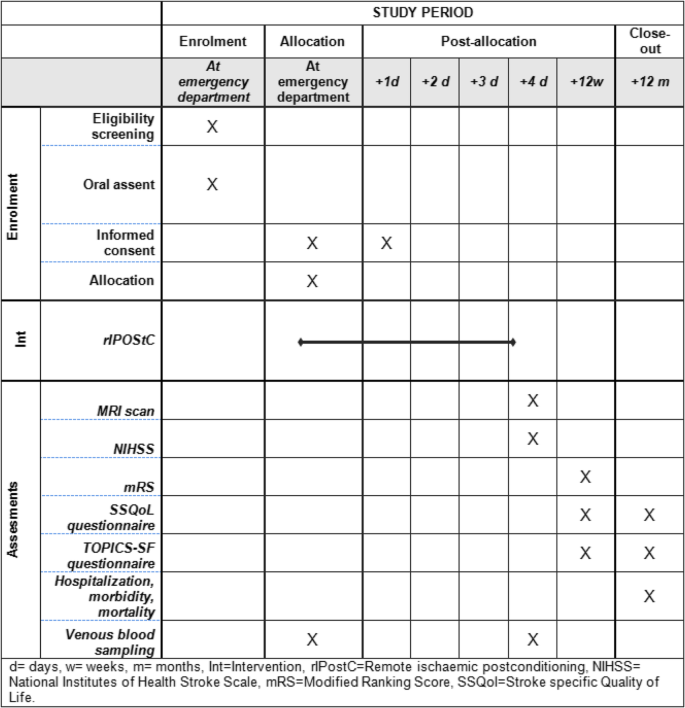
An overview of all outcomes and corresponding measurements is presented in Fig. 1.
Schedule of enrolment, interventions, and assessments according to the SPIRIT guidelines [40]. d day, w week, m month, Int intervention, MRI magnetic resonance imaging, mRS Modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, rIPostC Remote ischemic postconditioning, SSQoL Stroke-Specific Quality of Life, TOPICS-SF The Older Persons and Informal Caregivers Survey Short Form
No comments:
Post a Comment