Cessation of blood flow leads to a complex cascade of
pathophysiological events at the blood-vascular-parenchymal interface
which evolves over time and space, and results in damage to neural cells
and edema formation. Cerebral ischemic injury evokes a profound and
deleterious upregulation in inflammation and triggers multiple cell
death pathways, but it also induces a series of the events associated
with regenerative responses, including vascular remodeling,
angiogenesis, and neurogenesis. Emerging evidence suggests that
epigenetic reprograming could play a pivotal role in ongoing post-stroke
neurovascular unit (NVU) changes and recovery. This review summarizes
current knowledge about post-stroke recovery processes at the NVU, as
well as epigenetic mechanisms and modifiers (e.g., DNA methylation,
histone modifying enzymes and microRNAs) associated with stroke injury,
and NVU repair. It also discusses novel drug targets and therapeutic
strategies for enhancing post-stroke recovery.
Introduction
Stroke is defined as an abrupt onset of focal or global
neurological symptoms caused by a blockage of cerebral vessels (ischemic
stroke), rupture of blood vessels (hemorrhage), or transient occlusion
of small blood vessels (transient ischemic attack). It is particularly
prevalent in the aging population, with people over 65 years old
accounting for ∼75% of all registered cases (
Hollander et al., 2003).
Ischemic stroke is further subdivided based on the caliber of occluded
vessels into macro- and microvascular (i.e., lacuna stroke), and based
on the origin of clot-causing blockage into (a) thrombotic stroke where
clot form inside brain blood vessels, and (b) thromboembolic/embolic
stroke where clots form elsewhere in the body and travel toward and
lodge in brain blood vessels (
Reed et al., 2014;
Topcuoglu et al., 2018;
Tsai et al., 2018).
Pathophysiologically, cessation of blood flow leads to a
complex cascade of events at the blood-vascular-parenchymal interface
which evolves over time and space, and results in damage to neural cells
and edema formation (
Dirnagl et al., 1999;
Allen et al., 2012).
The central events in the hyperacute phase (within minutes and up to 6
h) include compromised mitochondrial function, anaerobic glycolysis,
decreased pH (acid condition), impaired ATP production and reduced ion
pump activity. As a consequence, cells swell and die, predominantly due
to accumulation of lactic acid, ions (Ca
2+ and Na
+) and increased water influx (
Dirnagl et al., 1999;
Ginsberg, 2008;
Nagy and Nardai, 2017).
There follows a cascade of events in the acute and
subacute phase (hours to 7 days), including blood-brain barrier (BBB),
and neurovascular unit (NVU) damage characterized by mitochondrial
failure, robust production of reactive oxygen species (ROS) (superoxide O
2,
hydrogen peroxide, and peroxynitrite), excitotoxicity (release of
glutamate from dying neurons), activation of matrix metalloproteinases
(MMP2, astrocytes; MMP3, MMP9 endothelial cells and neutrophils), BBB
damage triggering inflammation and blood cell infiltration (neutrophils,
monocytes) that can lead to further cell death, and cellular and
vasogenic edema (
Enzmann et al., 2013;
Posada-Duque et al., 2014;
Sifat et al., 2017).
The secondary damage mostly takes place in the penumbra surrounding the
core infarct and its progression can extend into the chronic phase
after stroke.
Paralleling these injury processes, there is activation
of endogenous protective and repair mechanisms that include vascular
remodeling, angiogenesis, and neurogenesis (
Chopp et al., 2007;
Venna et al., 2014).
The degree of these ongoing repair processes on one side and persistent
inflammation/damaging processes on the other determines stroke recovery
and the potential risk of another stroke.
There is mounting evidence on the importance of
epigenetic factors in stroke. The purpose of this review is to examine
how such factors impact the cerebrovasculature in stroke injury and
recovery, focusing on effects at the BBB, and NVU. There is debate over
whether non-coding RNAs should be included as an epigenetic mechanism
and this review will cover their effects as well as other epigenetic
mechanisms.
The Blood-Brain Barrier and Neurovascular Unit in Stroke
The blood-brain barrier is a highly complex and dynamic
barrier, formed by an interdependent network of brain capillary
endothelial cells, endowed with barrier properties. The BBB strictly
regulates paracellular permeability due to the presence of tight
junctions (TJs) between endothelial cells. Those TJs are built by
intricate interactions between transmembrane proteins (claudins -5, -3,
-1, -12, occludin, and JAM-A), important for paracellular space
occlusion, scaffolding proteins (ZO-1, -2), and the actin cytoskeleton
vital for physical support and TJ function (
Daneman et al., 2010;
Stamatovic et al., 2016). The transcellular interactions of claudin-5 play the major role in occluding the paracellular space (
Nitta et al., 2003;
Ohtsuki et al., 2007).
Any loosening of its adhesive interactions directly affects BBB
integrity and increases paracellular permeability. The BBB role of other
claudins with lower expression is still uncertain and under
investigation (
Tietz and Engelhardt, 2015;
Sladojevic et al., 2019).
BBB function is also dependent on the perivascular microenvironment,
which contains cells (e.g., pericytes, astrocytes, and perivascular
macrophages), neuronal endings and tissue unique extracellular matrix (
Mae et al., 2011;
Muoio et al., 2014).
Because of this functional integration, nearly two decades ago, the
concept of a BBB was broadened to a new structure, the NVU.
The neurovascular unit is composed of BBB-endowed
endothelial cells and a perivascular milieu composed from cells
including pericytes, smooth muscle cells, astrocytes, perivascular
macrophages/microglia, neurons/neuronal endings, and extracellular
matrix. The NVU mediates neurovascular coupling, modulating vessel tone (
Mae et al., 2011;
Muoio et al., 2014).
These intimately and reciprocally linked cells and matrix generate a
complex structure that regulates exchange between blood and brain,
oxygen and nutrient delivery, and regional cerebral blood flow. It is
essential for maintaining circulatory and brain homeostasis.
In stroke, blood-brain barrier, and neurovascular unit
dysfunction actively contributes to injury pathogenesis, being a “solid
substrate” for ongoing injuring processes (oxidative stress,
inflammation, and cytotoxicity), contributing to ischemic core (infarct)
formation in the acute phase of stroke, and facilitating the
progression of injury in the subacute and chronic phases. For example,
in the early (acute) phase of stroke, NVU dysfunction is characterized
by disruption of BBB integrity/BBB breakdown (disassembly of TJ complex,
decreases in the TJ proteins claudin-5, occludin, and ZO-1) that leads
to vasogenic brain edema, a life-threatening acute stroke complication (
Bauer et al., 2010;
Zehendner et al., 2011;
Sladojevic et al., 2014).
The cell components of the NVU undergo a series of transformations
during injury. Brain endothelial cells, for example, are affected very
early by cytotoxic effects with dysfunction of ion channels and
transporters (e.g., Na
+-K
+-Cl
– cotransporter, and Na
+/H
+
exchanger), release of extracellular vesicles and conversion of brain
endothelial cells toward a proinflammatory and prothrombotic phenotype
due to upregulation of protease-activated receptor 1 (PAR-1), tissue
factor, and matrix metalloproteinases (MMPs) (
Zhu et al., 2008;
Bauer et al., 2010;
Yamashita and Abe, 2011;
Chen et al., 2015).
The proinflammatory phenotype of brain endothelial cells involves an
upregulation of endothelial adhesive molecules (ICAM-1, VCAM-1, P-, and
E- selectins) that guide leukocyte infiltration in the acute
inflammatory phase response and T and B cells infiltration in the late
phase (
Kleinschnitz et al., 2013;
Zhou et al., 2013;
Sladojevic et al., 2014).
Overall, inflammation is thought to worsen acute ischemic injury,
contributing to chronic focal inflammation and restricting functional
recovery. However, inflammation is also involved in tissue repair.
In the hyperacute phase after stroke, pericytes may be
involved in vasoconstriction, causing capillary occlusion (no-reflow
phenomenon), while later, by switching to pro-inflammatory phenotype,
they may enhance BBB permeability, and brain edema formation (
Hall et al., 2014;
Underly et al., 2017).
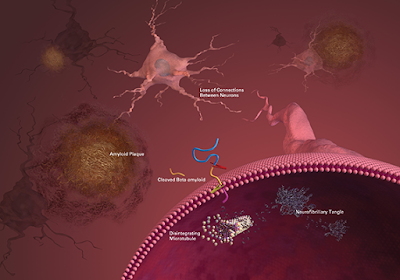
Ischemia triggers a series of damaging reactions in astrocytes
including mitochondrial dysfunction, energy depletion, ion
disequilibrium, increased glutamate and Ca
2+, aquaporin 4 (AQP4) channel activation, increased water permeability, and cell swelling (
Friedman et al., 2009;
Ito et al., 2009;
Hertz et al., 2014;
Mogoanta et al., 2014).
It results in the release of oxidative stress products and inflammatory
cytokines/chemokines (IL1, IL6, IL15, CCL2, CXCL1, CXCL10, CXCL12, and
IP-10), proinflammatory associated small molecules [S100 Ca
2+-binding protein B (S100B) and nitric oxide (NO)] that enhance the inflammatory post-stroke response (
Yamashita et al., 2000;
Hill et al., 2004;
Mori et al., 2008;
Shin et al., 2014;
Liu H. et al., 2015;
Chen et al., 2018).
Perivascular macrophages and microglia play an important
role in the stroke-induced inflammatory response via production of
proinflammatory cytokines (IL1|
upbeta TNFα IL6, IL12) and ROS (
Drake et al., 2011;
Liu H. et al., 2015;
Wu et al., 2016;
He et al., 2019).
They trigger the first line of inflammation at the NVU in the acute
phase of stroke. Notable changes also occur in the extracellular matrix.
At early time points (within hours), there is MMP-related basement
membrane degradation with reductions in agrin, SPARC, perlecan, laminin,
and fibronectin (
Sole et al., 2004;
Castellanos et al., 2007;
Lee et al., 2011;
Ji and Tsirka, 2012;
Lloyd-Burton et al., 2013).
This ultimately leads to increased BBB disruption, accumulation of new
extracellular matrix proteins (i.e., chondroitin sulfate proteoglycan
neurocan and osteopontin) and leakage of plasma proteins, such as
fibrinogen, into the CNS. This mediates inflammation, edema, and
potentially hemorrhagic transformation (
Figure 1).
More at link.



 Svetlana M. Stamatovic
Svetlana M. Stamatovic Chelsea M. Phillips
Chelsea M. Phillips Gabriela Martinez-Revollar1,
Gabriela Martinez-Revollar1,  Anuska V. Andjelkovic
Anuska V. Andjelkovic
 Older ‘gym rats’ might very well be staving off age-related cognitive impairment, researchers reported.
Older ‘gym rats’ might very well be staving off age-related cognitive impairment, researchers reported.
 Joshua Silverstein
Joshua Silverstein Katherine Zoe Tsagaris
Katherine Zoe Tsagaris Pasquale Fonzetti
Pasquale Fonzetti Rajiv R. Ratan
Rajiv R. Ratan Tomoko Kitago
Tomoko Kitago Marco Iacoboni
Marco Iacoboni Bruce Dobkin
Bruce Dobkin Dylan J. Edwards
Dylan J. Edwards