Stroke
is a leading cause of death and disability in the UK, and about 50% of
people who survive a stroke require some form of rehabilitation to
reduce impairment and assist with activities of daily living [1–3].
Upper limb function is particularly important in regaining independence
following stroke; impairments impact on daily living and well-being [4, 5].
Research
has consistently identified treatment intensity and goal oriented
strategies as critical elements for successful therapeutic outcomes [6–10].
To further maximise rehabilitation effects, novel therapeutic and
cost-effective rehabilitation interventions need to be developed and may
combine different methodological techniques. For example, the combined
use of electrical stimulation (ES), robot-aided therapy and virtual
reality (VR) environments has been suggested to be particularly
promising with respect to upper limb rehabilitation in chronic stroke [10, 11].
Following stroke, robot and ES therapies have been demonstrated to reduce upper limb motor impairments [6, 7, 10, 12–14]. Furthermore, these techniques have been highlighted as a way to facilitate the intensity of the training received [10],
and allow training despite muscle weakness and without the aid of a
therapist. In addition, when used with a real-time system which displays
the participants’ arm and hand movements in a VR environment, the
practiced movements can be very task-specific [11, 15].
These types of technologies may be more easily transferred into
patients’ homes, increasing the intensity and task specificity of the
training and reducing the time and expense constraints on therapists [16].
The therapeutic effect of ES in rehabilitation is known to increase when associated with a person’s voluntary effort [12].
However, a disadvantage of many ES approaches is that they fail to
encourage voluntary contribution. In addition, the vast majority of
upper limb stroke patient trials using ES employ open-loop or triggered
controllers [12, 17],
which can lead to imprecise control of movement. In the few cases that
closed-loop control has been employed, a simplistic structure and lack
of a model means accurate performance is still rarely achieved [18].
Employed mainly with spinal cord injury patients, one of the few
advanced control methodologies used comprises artificial neural networks
[19, 20].
However such model-free approaches have limited ability to adapt to
changing physiological conditions, must be re-trained for use with
different movements, and being of a “black-box” structure, do not permit
stability and performance analysis.
The study reported in this
paper investigates the feasibility and effectiveness of a novel 3D
rehabilitation platform which combines robotic support, ES and VR. The
system allows patients to receive the benefits of muscle-specific
targeted ES within a tightly controlled, safe and motivating
environment. In this platform, ES is mediated by iterative learning
control (ILC), a technology transferred from industrial robotics which
is applicable to systems which repeatedly perform a finite duration
tracking operation [21].
After each repetition, ILC uses data gathered on previous executions of
the task, often in combination with a model of the underlying system,
to update the ES signal that will be applied on the subsequent trial.
Hence ILC learns from previous experience the stimulation which
maximises performance, and can effectively respond to changes in the
model. ILC calculates the required control action in an optimal setting,
allowing strict regulation of the amount of ES, its trial-to-trial
variation, and the resulting movement error. Through use of appropriate
weighting parameters a precise balance can be placed between encouraging
voluntary effort and ensuring accurate movement [22, 23].
ILC is one of very few model-based upper limb ES control methodologies that has previously been used in a clinical study [24–26].
During this study, stroke participants attended 18 intervention
sessions of 1 hour duration in which they practiced planar reaching
tasks, tracking a moving spot of light. These movements were assisted by
ILC mediated ES applied to the triceps of the impaired arm. Unassisted
tracking performance (i.e., movements without the aid of ES) improved
over the course of the intervention and changes in muscle activation
patterns towards those of unimpaired participants were also observed [24, 25].
Whilst establishing the feasibility of advanced upper limb ES control
approaches in the clinical domain, this planar system did not assist
shoulder movement and by providing full mechanical support to the
forearm, allowed very limited shoulder elevation.
To address these
limitations and increase the potential of this novel approach to stroke
rehabilitation, a new system has been developed to assist participants
in performing more functional, 3D reaching tasks with ES applied to
triceps and anterior deltoid muscles [22, 23].
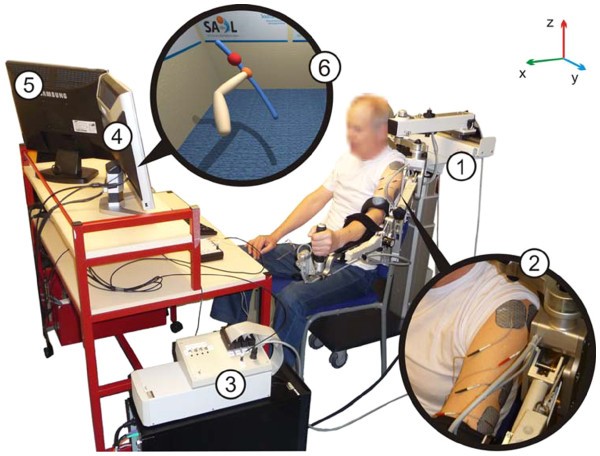
Termed SAIL: Stimulation Assistance through Iterative Learning, this
system comprises a commercial robotic arm support interfaced with
custom-designed ES hardware and real-time ES control environment,
together with a custom-made VR task display system (see Figure 1).
The
commercial exoskeleton robot is a purely passive ‘un-weighing’ system
which supports the patient’s arm against gravity via two springs
incorporated into the mechanism. Each of its joints contains a resolver
which records its angular position and this information is used by both
the ES control system, and the VR task display. Whilst building on
previous work, the ES controller incorporates substantial developments
in terms of biomechanical modelling, identification, and control
complexity compared with the planar system previously reported. In
particular, a five degree-of-freedom biomechanical model of the combined
human and robotic arm system was developed, along with identification
procedures using kinetic, kinematic and ES input data which are suitable
for patients [23, 27].
Then parallel feedback and feedforward controllers were derived using
techniques from nonlinear optimisation to achieve robust tracking whilst
maintaining strict trial-to-trial bounds on the change in input, and
the patients’ arm dynamics occurring along each trial [22, 23, 28, 29].
Moreover, the muscle structures used in the model, identification
procedure and controller have been specifically developed for
application to stroke patients [27].
Preliminary
tests to assess whether the ILC algorithms were accurately mediating
the ES took place with unimpaired participants. Results confirmed that
SAIL was effective in moving the arm to produce precise reaching
movements, and that tracking performance improved over a series of
trials see [22, 28, 29].
The aim of the study reported in this article was to assess the
technological feasibility and rehabilitation effectiveness of the SAIL
system with chronic stroke participants.
More at link.
 Muhammad Ahmed Khan
Muhammad Ahmed Khan Iris C. Brunner
Iris C. Brunner Maarten G. Lansberg
Maarten G. Lansberg Ada Poon
Ada Poon Kimford J. Meador
Kimford J. Meador