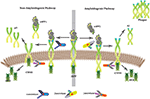
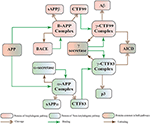
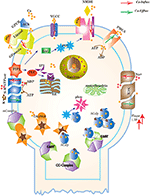
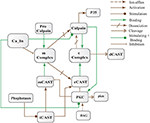
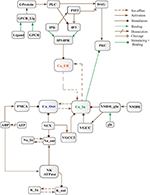
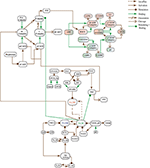
Abstract
Objectives
This
study examines the relationship between alcohol consumption and
incident stroke among older adults and tests whether alcohol consumption
contributes to observed race and sex differences in stroke.
Method
Data
are from a U.S. national cohort of black and white adults aged 45 and
older, the REasons for Geographic And Racial Differences in Stroke
(REGARDS) study. Current and past drinking levels were reported at
baseline (2003–2007). Participants who had never had a stroke were
followed for adjudicated stroke events through September 2015 (n
= 27,265). We calculated Cox proportional hazard models for stroke,
adjusting for demographic, socioeconomic, behavioral, and health
characteristics.
Results
Participants,
mean age 64.7 years, consumed on average 2.2 drinks/week and
experienced 1,140 first-time stroke events over median 9.1 years
follow-up. Nondrinkers had a 12% higher risk of stroke than current
drinkers; the risk of stroke among nondrinkers largely reflected high
risks among past drinkers; these differences were explained by
socioeconomic characteristics. Among current drinkers, light drinkers
had significantly lower stroke risks than moderate drinkers after
accounting for demographic, socioeconomic, behavioral, and health
characteristics. Implications of alcohol did not differ between blacks
and whites but did differ by sex: Especially among women, nondrinkers,
and specifically past drinkers, had higher risks; these differences were
largely explained by health characteristics and behaviors. Alcohol did
not explain race and sex differences in stroke incidence.
Discussion
Among
older adults, those who used to, but no longer, drink had higher risks
of stroke, especially among women; current light drinkers had the lowest
risk of stroke.
Writing during the prohibition era, Raymond Pearl concluded that
moderate consumption of alcohol was not harmful to one’s health (
Pearl, 1926).
Since then, several studies have indicated that moderate consumption of
alcohol is associated with better health and lower mortality risks. The
relationship between alcohol consumption and mortality has been
described as U shaped, with higher mortality for abstainers and heavy
drinkers and lower mortality for moderate drinkers (
Marmot, Rose, Shipley, & Thomas, 1981).
Moderate alcohol consumption has been found to be inversely associated
with coronary heart disease morbidity and mortality across several
populations (
Marmot, 1984).
Some scholars have argued that the robustness of these associations
across methods and populations indicates protective effects of alcohol
consumption against heart disease and mortality (
Bovet & Paccaud, 2001;
Marmot, 2001).
Few
studies have examined the health implications of alcohol consumption
for older adults, but there is some evidence that moderate alcohol
consumption is also linked with lower mortality at older ages (
Goldberg, Burchfiel, Reed, Wergowske, & Chiu, 1994;
Thun et al., 1997). For adults, especially at older ages, stroke is a major cause of morbidity and mortality (
Howard & Goff, 2012;
Mozaffarian et al., 2015). This study examines the relationships between alcohol consumption among older adults and their risk of stroke.
Alcohol Consumption Patterns
In the United States in 2012, 71% of adults aged 18 years and older
reported drinking in the past year, and 51.3% of adults were current
regular drinkers, defined as 12 or more drinks in the past year (
Schiller, Lucas, Ward, & Peregoy, 2012). Older adults tend to decrease their total alcohol intake after retirement (
Ferreira & Weems, 2008); in a 2008 national survey, approximately 40% of adults aged 65 years and older reported that they drank alcohol (
Jardim-Botelho et al., 2014).
Alcohol consumption patterns differ by sex and race (
Petrea et al., 2009;
Rosamond et al., 1999). Men tend to drink more frequently and in larger amounts than women, and women are more often lifetime abstainers (
Wilsnack, Vogeltanz, Wilsnack, & Harris, 2000); these patterns are similar for adults aged 65 years and older (
Ferreira & Weems, 2008). Whites are more likely to drink alcohol, but blacks who drink have higher volume of intake and frequency of heavy drinking (
Chartier & Caetano, 2010;
Fesahazion, Thorpe, Bell, & LaVeist, 2012;
Kerr, Patterson, & Greenfield, 2009).
Fewer black than white men are heavy drinkers; however, those black men
who are heavy drinkers tend to maintain heavy drinking practices to
older ages (
Chartier & Caetano, 2010).
Alcohol Consumption and Health
Several studies have shown links between alcohol consumption,
morbidity, and mortality. Even after controlling for numerous possible
confounders, such as age, employment, race, smoking, blood pressure,
body mass index (BMI), fat consumption, and cholesterol, the
relationship between alcohol consumption and mortality is U shaped (
Fuller, 2011;
Liao, McGee, Cao, & Cooper, 2000;
Marmot et al., 1981;
Paganini-Hill, Kawas, & Corrada, 2007;
Thun et al., 1997;
Wannamethee & Shaper, 1997).
Studies have found U-shaped relationships between alcohol consumption
and cardiovascular disease (CVD) mortality in several populations (
Fuller, 2011;
Marmot, 1984;
Thun et al., 1997).
Data from the Cancer Prevention II study of adults aged 30 years and
older showed that risk of death from coronary heart disease and other
circulatory diseases was lower for men and women who drank one to three
drinks per day compared with those who did not drink (
Thun et al., 1997).
Nationally representative U.S. data indicated that moderate drinkers
had lower all-cause and coronary heart disease mortality than
nondrinkers, even when adjusting for age, race, education, marital
status, employment, smoking, income, self-reported health, and previous
diagnosis of heart problems (
Fuller, 2011).
In the Whitehall Study of male civil servants aged 40–64 years in
England, CVD mortality was higher among nondrinkers than among drinkers (
Marmot et al., 1981).
Biological
mechanisms could underlie cardioprotective effects of moderate
drinking. Specifically, moderate drinking increases levels of
high-density lipoprotein cholesterol, which can prevent clots and reduce
platelet aggregation and so can protect against CVD and stroke (
Agarwal, 2002).
At the same time, inverse associations between alcohol intake and morbidity and mortality could be spurious (
Fillmore, Stockwell, Chikritzhs, Bostrom, & Kerr, 2007;
Thun et al., 1997).
For example, in the United States, nondrinkers often are from poorer
socioeconomic circumstances and have lower levels of education than
drinkers, and socioeconomic status and education are positively
associated with health; thus, the poorer health outcomes of nondrinkers
may be due to their socioeconomic disadvantage rather than their
avoidance of alcohol (
Fekjaer, 2013;
Fillmore et al., 2007;
Naimi et al., 2005;
Naimi, Xuan, Brown, & Saitz, 2013).
Therefore, it is important to examine associations using adequate
controls for socioeconomic status and to not rely on cross-sectional
associations. Another concern is that people may stop drinking precisely
because they are experiencing health problems, entailing possible
reverse causation. Thus, former drinkers may be at higher risk for
adverse health outcomes; occasional drinkers (less than 12 drinks/year)
may also include individuals who reduced their alcohol intake due to
health problems. Therefore, former drinkers, occasional drinkers, and
lifetime abstainers should be separated in analyses, as combining them
may show artificially high risks for nondrinkers. Some of the documented
protective effects of alcohol may disappear when former drinkers and
occasional drinkers are separated from lifetime abstainers (
Fillmore et al., 2007).
In addition, consequences may differ for those drinking seven drinks
over the course of a week or over the course of one day, so patterns of
drinking should be considered (
Marmot, 2001;
Thun et al., 1997).
Nonetheless, several studies showed that moderate alcohol consumption
was associated with better outcomes, even after controlling for
socioeconomic status and distinguishing former and occasional drinkers
from lifetime abstainers. They have shown a U-shaped relationship
between alcohol consumption and all-cause mortality, coronary heart
disease mortality, and intracerebral hemorrhage (
Thrift, Donnan, & McNeil, 1999).
Patterns of Stroke and Associatons With Alcohol
There are two major types of stroke: (a) ischemic stroke accounts for
the majority of strokes in the United States and occurs as a result of
an obstruction in a blood vessel supplying blood to the brain; (b)
hemorrhagic stroke occurs when a weakened blood vessel ruptures. The
association between alcohol consumption and stroke may vary with type of
stroke (
Klatsky, 2015).
Across
case–control and cohort studies, ischemic stroke morbidity and
mortality had J-shaped relationships with alcohol consumption (
Camargo, 1996;
Patra et al., 2010).
A meta-analysis of 35 studies found lower risks of stroke for drinkers
who consumed ≤12 g of alcohol per day compared with abstainers (
Reynolds et al., 2003):
light drinkers had a 17% lower risk of total stroke and 20% lower risk
of ischemic stroke compared with abstainers; moderate drinkers (12–23
g/day) also had a 25% lower risk of ischemic stroke compared with
abstainers.
Hemorrhagic stroke morbidity and mortality increased with alcohol use (
Camargo, 1996;
Patra et al., 2010), with a positive linear relationship between alcohol consumption and hemorrhagic stroke (
Reynolds et al., 2003).
Although heavy drinking is associated with hemorrhagic stroke, the
relationships between light-to-moderate alcohol consumption and
hemorrhagic stroke have been conflicting, likely because of small
numbers of hemorrhagic strokes in most studies (
Owolabi & Agunloye, 2013;
Patra et al., 2010;
Thrift et al., 1999).
Another
meta-analysis of 27 prospective studies found that light drinkers had a
lower risk of total stroke, ischemic stroke, and stroke mortality but
not hemorrhagic stroke; heavy drinkers had higher risk of total stroke
but not of hemorrhagic stroke, ischemic stroke, or stroke mortality (
Zhang et al., 2014).
In the ARIC study of older adults across four U.S. communities, light
and moderate drinkers did not have a lower incidence of ischemic stroke
than abstainers, whereas heavy drinkers had higher incidence (
Jones et al., 2015).
In
a prospective cohort of Swedish older adults, those who had been very
light drinkers (<0.5 drink/day) in middle age had significantly lower
risks of stroke during the following four decades than did heavy
drinkers (>2 drinks/day) and nondrinkers; the risk of stroke among
nondrinkers increased with age, whereas the risks associated with heavy
drinking decreased (
Kadlecová, Andel, Mikulík, Handing, & Pedersen, 2015).
Stroke incidence and mortality differ between blacks and whites in the United States (
Gillum, 1999;
Go et al., 2014;
Howard et al., 2011;
Kleindorfer et al., 2010;
Sacco et al., 1998):
black men have the highest age-adjusted rate of incident stroke
(4.4/1,000 person-years), black women the second highest (3.1/1,000
person-years), and white men (1.8/1,000 person-years) and women the
lowest (1.2/1,000 person-years;
Rosamond et al., 1999). Stroke mortality rates follow similar patterns (
Gillum, 1999).
Whether
the relationship between alcohol consumption and stroke outcomes
differs for men and women remains uncertain. Some studies have reported
lower risks of incident stroke among drinkers for both men and women,
but different patterns with respect to stroke mortality (
Ikehara et al., 2008;
Zheng et al., 2015). Others have reported protective effects only for women (
Hansagi, Romelsjo, Gerhardsson de Verdier, Andreasson, & Leifman, 1995);
still others have reported that women experience a J-shaped
relationship rather than linear relationship for hemorrhagic stroke (
Jimenez et al., 2012).
This
study examined the relationships between alcohol consumption among
older adults and their risk of experiencing a stroke. As previous
research identified higher risk of stroke among blacks compared with
whites and among men compared with women, as well as differences in
alcohol consumption patterns across these groups (
Fesahazion et al., 2012;
Go et al., 2014;
Howard et al., 2011;
Kleindorfer et al., 2010;
Petrea et al., 2009;
Sacco et al., 1998),
we explore whether differences in alcohol consumption explain some of
the observed differences in stroke risks between blacks and whites and
between men and women.
Method
Data
We used data from the REasons for Geographic and Racial Differences
in Stroke (REGARDS) study, a national longitudinal study of black and
white adults aged 45 years and older (
n = 30,239). Stroke risks differ across regions of the United States (
Borhani, 1965;
Howard et al., 2011), and the REGARDS study was designed to measure and understand these differences (
Howard et al., 2005).
Therefore, a stratified random sample was conducted with oversampling
in the region dubbed the “stroke belt” (Alabama, Arkansas, Georgia,
Louisiana, Mississippi, North Carolina, South Carolina, Tennessee;
Lanska & Kuller, 1995).
Twenty-one percent of the sample was randomly selected from the
“buckle” of the stroke belt (coastal plain region of North Carolina,
South Carolina, and Georgia), 35% from the rest of the stroke belt
states (remainder of North Carolina, South Carolina, and Georgia plus
Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and the
remaining 44% from the other 40 contiguous U.S. states. Blacks were
oversampled to characterize racial differences in stroke.
Participants
were recruited between January 2003 and October 2007. Each participant
was first mailed a letter and brochure explaining the study and then
telephoned to recruit and obtain verbal consent. Written consent was
obtained during the subsequent in-person evaluation. Using a
computer-assisted telephone interview (CATI), trained interviewers
obtained information on demographic and socioeconomic characteristics,
medical history, and lifestyle risk factors. A brief physical exam,
including blood pressure measurements, blood samples, and anthropometry,
was conducted in-person 3–4 weeks after the CATI. Participants were
contacted every 6 months by telephone to document self- or
proxy-reported suspected stroke. The institutional review boards of
participating institutions approved the study. Additional details on
REGARDS are provided elsewhere (
Howard et al., 2005).
REGARDS
cooperation and response rates at baseline were 49% and 33%,
respectively, comparable with other cardiovascular cohort studies (
Morton, Cahill, & Hartge, 2006).
During follow-up, more than 80% of participants completed at least 75%
of follow-up. The REGARDS study is still ongoing, but, for this
analysis, we used follow-up data through September 2015.
Respondents who had reported having ever experienced a stroke at baseline were excluded from this analysis (
n = 1,930), as were those missing in-person data (
n = 56), those who only participated in the baseline questionnaire (
n = 447), and those who did not respond to questions about alcohol (
n = 541), resulting in an analytic sample of 27,265.
Alcohol Determination
Data on alcohol use were collected through questions at baseline. The
first was “Do you presently drink alcoholic beverages, including beer,
wine, and other drinks made with hard liquor, even occasionally?” If
answered affirmatively, the following question was asked: “How many
alcoholic beverages do you presently drink? For example, one per day,
three per week, and so on. Please include beer, wine and hard liquor.”
If participants answered “no” to the first alcohol question, the
follow-up question was “Have you ever drunk alcoholic beverages,
including beer, wine, and other drinks made with hard liquor on a
regular basis? By regular, we mean at least 1 drink per month for 1
year.”
We created three measures of alcohol consumption. The
simplest was current drinking status: drinker or not drinker. A second
measure was drinking history: current drinker, lifetime abstainer, or
past regular drinker. The third measure was consumption level, which
additionally categorized current drinkers, in line with National
Institute on Alcohol Abuse and Alcoholism definitions, as occasional
drinkers (current drinkers who did not drink in an average week), light
drinkers (up to 1 drinks/week on average for both men and women),
moderate drinkers (1–7.5 drinks/week for women and 1–15 drinks/week for
men), and heavy drinkers (≥7.5 drinks/week for women and ≥15 drinks/week
for men;
National Institute on Alcohol Abuse and Alcoholism, 2016).
Stroke Events Determination
The outcome of interest was the occurrence of any adjudicated stroke
event in a person who reported never having had a stroke at baseline.
During telephone interviews at each follow-up contact, a report of
possible stroke, transient ischemic attack, death, hospitalization or
emergency department visit for brain aneurysm, brain hemorrhage, stroke
symptoms, or unknown reason generated a request for retrieval of medical
records. A stroke nurse conducted an initial review to exclude events
that were obviously not strokes. Then, medical records of suspected
strokes were centrally adjudicated by physicians. For deaths with no
medical records, death certificates and/or proxy interviews were used.
Stroke was defined using the World Health Organization (WHO) definition
of focal neurologic symptoms lasting more than 24 hr or those with
neuroimaging data consistent with stroke. Details of this method are
described elsewhere (
Howard et al., 2011).
The outcome variable combined clinical and WHO stroke definitions.
Strokes were classified as ischemic or hemorrhagic whenever the type
could be determined.
Covariates
Self-reported characteristics at baseline were used as covariates in
models. Demographic characteristics were: age, race (black, white), and
sex (female, male). Social and economic variables were urbanicity of
residence (urban, rural, or mixed—county-level category from the 2000
U.S. Census), annual household income (<$20,000, $20,000–$34,000,
$35,000–$74,000, >$75,000), education level (less than high school,
high school graduate, some college, college graduate and above), and
marital status (married, divorced, widowed, single, other). Stroke belt
residence is a socioeconomic indicator as well as a sampling criterion
(stroke belt, stroke buckle, non-belt). Health and health behaviors
measures were smoking (yes, no), physical activity (≥4 times/week, 1–3
times/week, 0 times/week—in response to a question about frequency of
engaging in intense physical activity sufficient to work up a sweat),
BMI category (underweight, normal, overweight, obese—based on measured
height and weight compared with standard CDC cut points), diabetes (yes,
no—based on fasting glucose ≥ 126 mg/dL, nonfasting ≥ 200 mg/dL, or
self-report of glucose control medication), and hypertension (yes,
no—based on systolic blood pressure ≥ 140 mmHg, diastolic blood pressure
≥ 90 mmHg, or self-reported current hypertension medication use).
Statistical Analysis
The follow-up period for analysis was from recruitment until
September 30, 2015. Follow-up time for each participant was calculated
from date of in-home visit to date of first stroke, death, or last
telephone contact. Similarly, attained age was calculated from date of
birth to date of first stroke, death, or last telephone contact.
Demographic, social, economic, health, and behavior characteristics at
baseline were examined for the entire cohort and then compared across
current drinkers and nondrinkers. Continuous variables were summarized
as means and standard deviations, and statistical differences were
detected using
t tests. Categorical variables were summarized as proportions and tested for significant differences using chi-square tests.
Cox
proportional hazard models were used to estimate the associations
between incident stroke and the three measures of alcohol exposure (
Table 2),
first unadjusted (Model 0) and then sequentially adding demographic
characteristics (Model 1), social and economic characteristics (Model
2), and health characteristics and behaviors (Model 3). Attained age was
used as the time variable in models.
To determine whether the
implications of alcohol consumption were different for blacks compared
with whites and for men compared with women, we tested two-way and
three-way interaction terms between race and sex and the three measures
of alcohol use. Significance of interaction terms was examined using an a
priori level of α < .10, which indicates heterogeneity in risk
justifying stratified models. Stratified results are presented in
Table 3.
As
previous research has identified higher risk of stroke among blacks
compared with whites and among men compared with women, as well as
differences in alcohol consumption patterns across these groups, we
examined whether patterns of alcohol consumption explained these
differences in risks of stroke (
Table 4).
Finally,
because the relationships between alcohol and stroke type may differ,
we also present stratified models examining relationships between
alcohol consumption and ischemic and hemorrhagic stroke (
Table 5).
Analyses
were conducted using SAS 9.3. The proportional hazards assumption was
tested by including the cross-product of log-transformed age and each of
the covariates in the final Cox models.
Results
Drinking patterns are shown in
Table 1.
Nearly half (48.2%) of the participants were not current drinkers; most
nondrinkers (63.3%) were lifetime abstainers. Among current drinkers,
more than a third were moderate drinkers, 28.6% were light drinkers,
26.5% were occasional drinkers, and less than 8% were heavy drinkers.
More at link.
 Javaria Ashraf
Javaria Ashraf Jamil Ahmad
Jamil Ahmad Amjad Ali
Amjad Ali Zaheer Ul-Haq
Zaheer Ul-Haq