No clue what this means, too many big words used to confuse stroke survivors. So you'll have to ask your doctor if this will get you 100% recovered since nothing about full recovery was ever discussed.
Assisting walking balance using a bio-inspired exoskeleton controller
Journal of NeuroEngineering and Rehabilitation volume 20, Article number: 82 (2023)
Abstract
Background
Balance control is important for mobility, yet exoskeleton research has mainly focused on improving metabolic energy efficiency. Here we present a biomimetic exoskeleton controller that supports walking balance and reduces muscle activity.
Methods
Humans restore balance after a perturbation by adjusting activity of the muscles actuating the ankle in proportion to deviations from steady-state center of mass kinematics. We designed a controller that mimics the neural control of steady-state walking and the balance recovery responses to perturbations. This controller uses both feedback from ankle kinematics in accordance with an existing model and feedback from the center of mass velocity. Control parameters were estimated by fitting the experimental relation between kinematics and ankle moments observed in humans that were walking while being perturbed by push and pull perturbations. This identified model was implemented on a bilateral ankle exoskeleton.
Results
Across twelve subjects, exoskeleton support reduced calf muscle activity in steady-state walking by 19% with respect to a minimal impedance controller (p < 0.001). Proportional feedback of the center of mass velocity improved balance support after perturbation. Muscle activity is reduced in response to push and pull perturbations by 10% (p = 0.006) and 16% (p < 0.001) and center of mass deviations by 9% (p = 0.026) and 18% (p = 0.002) with respect to the same controller without center of mass feedback.
Conclusion
Our control approach implemented on bilateral ankle exoskeletons can thus effectively support steady-state walking and balance control and therefore has the potential to improve mobility in balance-impaired individuals.
Introduction
Wearable robotic devices (e.g. exoskeletons and prostheses) are currently being developed to enhance mobility in able-bodied subjects, or to restore mobility in persons with musculoskeletal and neurological disorders. Recent developments in hardware and control enabled large reductions in muscle activity and metabolic energy consumption in able-bodied subjects during walking [1,2,3,4,5]. Nevertheless, researchers still struggle to design controllers for wearable robotic devices that not only reduce effort, but also support balance control. The limited ability to support balance has important negative implications as individuals with mobility impairments typically have reduced balance control resulting in a high fall incidence [6,7,8]. Many potential users would potentially benefit from wearable robotic devices that simultaneously reduce effort and support balance during walking. Here, we developed and tested a biomimetic controller for a bilateral ankle exoskeleton that simultaneously supports steady-state and perturbed walking.
Commonly used exoskeleton controllers that prescribe kinematics or assistive moments are not easily extendable to include balance control as flexible balance control requires online feedback from the state of the human and exoskeleton. Traditionally, exoskeletons are controlled using pre-defined joint angle [9] or torque trajectories [1]. Control approaches based on torque trajectories have been very successful in reducing metabolic cost in healthy users [1,2,3,4,5]. As these control approaches do not provide balance support, balance impaired users often have to use external stabilizers such as crutches or walkers that limit function [7]. For applications in spinal cord injury patients where the exoskeleton takes over from the user, some lower limb exoskeletons that support balance have been developed [10]. However, such exoskeletons only support walking using a control strategy that is not necessarily compatible with the human balance control strategy. Two recent studies evaluated exoskeleton controllers that cooperate with the balance control of the user by imposing a predefined torque trajectory that mimics the joint moment in response to perturbations in healthy subjects [11, 12]. These studies demonstrated the potential of exoskeletons to support balance. Yet, this control approach is not generalizable to different types of perturbations. Notably, the adaptability and stability of human locomotion originates from sensorimotor feedback.
Biomimetic exoskeleton controllers that are inspired by human reflex control during steady-state walking have been shown to be adaptable but they do not stabilize walking against whole-body perturbations. Geyer and Herr proposed a computational model of the musculoskeletal system that produces stable walking based on local feedback control [13]. In this model, both muscle dynamics and local feedback provide stabilization against local perturbations. This model has been used to control exoskeletons and prostheses [14,15,16,17]. In this case, the joint angles derived from encoders on the device are the input to the neuromuscular model whereas the joint moments are the outputs. The resulting controllers have been shown to be adaptable to different walking speeds [18]. However, local feedback control is not sufficient to stabilize human movement against whole-body perturbations. In simulation, Song & Geyer showed that the neuromechanical model can predict the main changes in muscle activity in response to local perturbations (e.g. mechanical tap of tendons), but not to whole-body disturbances such as slip and trip perturbations [19]. A neuromuscular controller for a transfemoral prosthesis leads to more robust walking in simulation compared to minimal impedance control, but does not capture human responses to mid-swing disturbances in hardware experiments [20]. These results might not be surprising given that humans use sensory integration to shape feedback responses and thus do not rely solely on local feedback.
Supra-spinal feedback pathways have an important contribution in human standing and walking balance control [21]. Changes in ankle muscle activity and moments after fore-afterward perturbations during standing and walking can be explained by delayed feedback of whole-body center of mass (COM) kinematics [22,23,24,25,26]. The relation between muscle activity and delayed COM kinematics also indicates that supra-spinal mechanisms, and not only local feedback loops, are important in human balance [21, 26, 27]. While humans cannot directly sense COM kinematics, it is assumed that sensory information is integrated and processed in the nervous system to estimate the state of the body and thus the COM. The relation between COM kinematics and ankle muscle activity in perturbation experiments therefore suggests that delayed feedback of COM kinematics is a simple but relevant model to describe the complex process of sensory integration and state estimation in the nervous system. Using feedback control from COM kinematics might also improve the ability of wearable robotic devices to support balance control. Such a biomimetic control strategy might result in an intuitive cooperation between the balance recovery actions of the user and exoskeleton.
We developed an ankle–foot exoskeleton controller that aimed at assisting both steady-state and perturbed walking. Inspired by the observed relation between COM kinematics and reactive muscle activity in perturbation experiments in humans [22], the proposed exoskeleton controller relies on feedback of COM kinematics in addition to local feedback of ankle kinematics to estimate the required ankle moment. We identified control parameters in the underlying neuromechanical model by fitting simulated and measured ankle joint moments in a dataset with steady-state walking and walking with pull and push perturbations applied at the pelvis [28]. The resulting neuromechanical model was used to control an ankle foot exoskeleton in a novel perturbation experiment. The exoskeleton provided 30% of the joint moment estimated by the model during steady-state and perturbed walking. Our main hypotheses were that additional feedback of COM kinematics is needed in a neuromechanical model to capture changes in joint moment after perturbations (i.e. to reduce the difference between simulated and measured ankle joint moment); and ankle–foot exoskeletons controlled with a neuromechanical model with additional feedback of COM kinematics will assist balance control. Given the interaction with the human, successful assistance might result in either reduced muscle activity and/or reduced deviations from steady-state locomotion in response to perturbations.
Results
Parameter identification perturbed walking
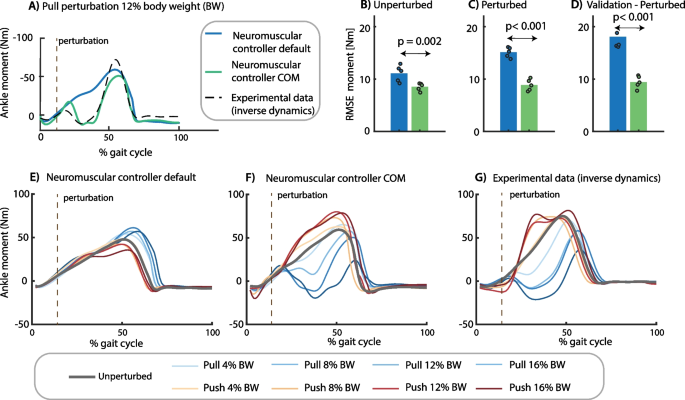
We first identified control parameters of a neuromechanical model based on an existing motion-capture dataset. This dataset documents the response to pull and push perturbations applied at the pelvis at toe-off during slow walking at 0.62 m/s [28]. We started from a state-of-the art neuromechanical model with virtual Hill-type muscles driven by local reflexes with ankle angles and ground reaction forces as input and joint moments as output (i.e. default neuromechanical model) [13]. We extended this model using additional feedback of deviations in COM velocity with respect to steady-state walking. Control parameters were estimated by optimizing the fit between simulated and measured ankle moments during steady-state and perturbed walking for both the default neuromechanical model and the neuromechanical model with COM feedback.
Adding COM velocity feedback was needed to track the ankle moment in perturbed walking (Fig. 1). The root mean square error (RMSE) in ankle joint moment was 14 Nm in the default neuromechanical model and 9 Nm in the model with COM feedback (Fig. 1B, C). The model with COM feedback can predict the decrease in ankle moment in response to pelvis pull and the increase in ankle moment in response to pelvis push perturbations. In contrast, the default model predicts an increase in ankle moment, opposite to the observed decrease, in response to pelvis pull perturbations and no change in ankle moment in response to pelvis push perturbations (Fig. 1D). Although we did not optimize the fit between measured and simulated muscle activity, the model with additional COM feedback also captured the increase in calf muscle activity in response to push perturbation and the increase in tibialis anterior activity in response to pull perturbations (Additional file 1: Fig. S2).
Control parameter identification. We estimated the control parameters of a model without (blue) and with (green) additional feedback of whole body center of mass (COM) velocity by minimizing the difference between the experimental ankle moment and the ankle moment simulated with the neuromechanical model (representative example in A). Control parameters of each model were estimated by tracking eight steady-state and 16 perturbed gait cycles in one optimization problem. Perturbations were applied at toe-off of the contralateral leg while the subjects walked at 0.62 m/s. The root mean square error (RMSE) between experimental and simulated ankle moments was smaller in the model with additional COM feedback for (B) steady-state walking, (C) perturbed walking, and (D) a validation perturbation trial that was not used in the parameter estimation (the dots represent the RMSE in each of the five subjects and the bar represents the average across subjects). The lower RMSE for the model with COM feedback compared to the default model reflects the simulated change in ankle moment in response to pelvis push and pull perturbations (F) that is in agreement with experimental observations (G), whereas the default reflex model without COM feedback cannot capture the experimental data (E). (E–G contains data of one representative subject with the two-trial average response of each unique perturbation)
We evaluated the parameter estimation results using cross-validation on novel trials at a perturbation magnitude not included in the parameter estimation. The RMSE between simulated and measured ankle moments was similar for the validation trials than for the trials used for estimation (Fig. 1D).
Experiment steady-state and perturbed walking with ankle exoskeleton
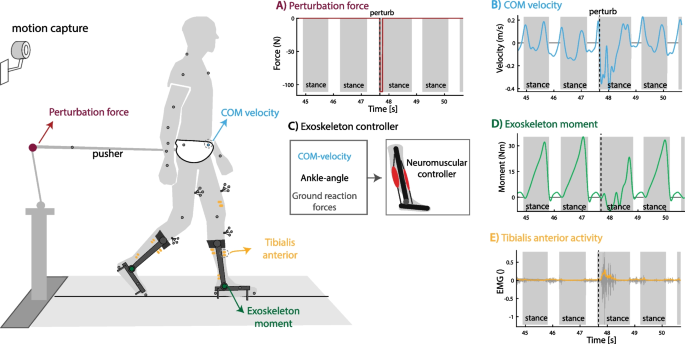
We implemented both controllers on a bilateral ankle–foot exoskeleton and compared them with a minimal impedance controller in twelve healthy participants during treadmill walking at 0.6 m/s. We selected this slow walking speed because many populations that would benefit from an exoskeleton typically walk slow even with assistive devices (e.g. frail elderly, partial spinal cord injury patients). A robotic pusher was used during the experiment to apply forward and backward directed forces to a waist belt worn by the participants (Fig. 2). The minimal impedance controller minimized the joint moments delivered by the exoskeleton using a disturbance observer [29]. We used the neuromuscular controller with and without COM feedback with the identified control parameters (average across five subjects) to estimate the ankle moment based on the ankle angle measured by the exoskeleton encoders, COM velocity estimated from the trajectory of a marker on the pelvis (motion capture; only for model with COM feedback), and ground reaction forces measured using an instrumented treadmill. The exoskeleton delivered 30% of the ankle joint moment computed with the neuromuscular model. The value of 30% was chosen to have a similar peak exoskeleton moment as in other experiments [2].
Perturbed walking with ankle–foot exoskeleton. Twelve subjects walked with a bilateral ankle–foot exoskeleton in minimal-impedance mode and controlled with the neuromechanical model with and without additional COM velocity feedback. A External forces were applied at the pelvis after right heel strike in forward (push) or backward (pull) direction to perturb human walking. B, C The neuromuscular controller uses the encoder on the ankle joint, ground reaction forces and deviation in COM velocity from the reference trajectory as input and ankle joint moment as output. D The exoskeleton delivered 30% of the ankle joint moment computed with the neuromuscular controller. E Surface Electromyography was used to quantify muscle activity to evaluate controller performance. This figure contains data of one backward directed perturbation of a typical subject walking with the neuromuscular controller with additional feedback of COM velocity
Muscle activity during unperturbed walking with exoskeleton
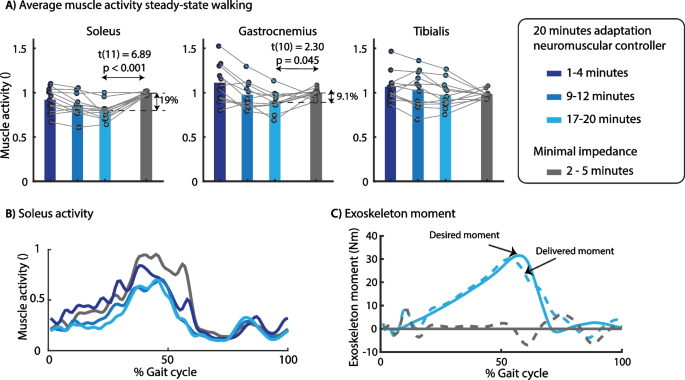
Subjects walked for 20 min with the default neuromuscular controller (without being perturbed) to adapt to treadmill walking with the exoskeleton. During this adaptation period, subjects changed their gait by reducing soleus activity (Fig. 3). After the adaptation period, the subjects walked for 5 min with the minimal impedance controller. On average, soleus activity was 19% lower (p = 0.001) when walking with the default neuromuscular controller than with the minimal impedance controller (Fig. 3A, B). The decrease in gastrocnemius activity was smaller compared to the soleus (9.1%, p = 0.045).
Effect of exoskeleton assistance on muscle activity in steady-state walking. We observed a gradual decrease in soleus muscle activity during the 20 min adaptation. Compared to minimal impedance mode (gray), average soleus activity was 19% lower at the end of the adaptation period (A). The soleus activity was decreased during the full duration of the stance phase (B), which is the result of the plantarflexion assistance provided by the exoskeleton (C). The desired exoskeleton moment was applied with a RMSE of 2.02 Nm with mainly larger differences between desired and actual moment during the first part of the stance phase. A paired t-test was used to compare muscle activity between controllers. A contains data of all subjects with the dots representing the median muscle activity during 3 min of walking and the bars the averaged data across subjects. The time series in B and C are based on data of one representative subject
Next, subjects walked for 5 min with each of the controllers while being perturbed by forward or backward directed external forces with a magnitude of 12% of body and exoskeleton weight and a duration of 200 ms applied to the pelvis. All perturbations were applied at right heel strike with randomized time intervals between perturbations. We analyzed and compared muscle activity in the last two minutes of each condition during perturbed and unperturbed gait cycles separately. During the unperturbed gait cycles, soleus activity was 20% lower with both neuromuscular controllers than with the minimal impedance controller (Additional file 1: Fig. S8). Hence, the neuromuscular controllers with and without COM feedback caused a similar reduction in soleus activity for unperturbed gait cycles, which is not surprising given the small variation in COM velocity during these gait cycles.
Exoskeleton assistance in perturbed walking
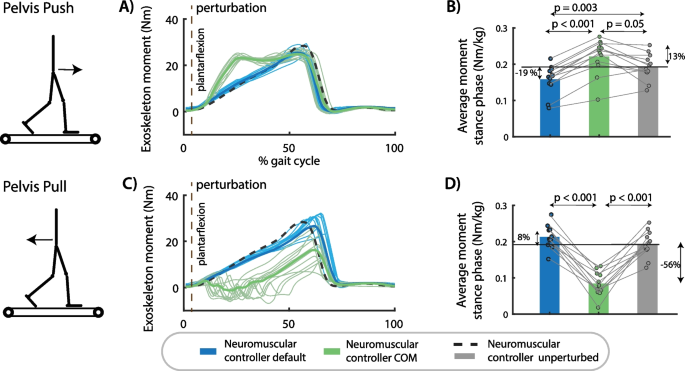
Assistive joint torques computed by both neuromuscular controllers during perturbed cycles were in agreement with the simulations. The joint moments delivered by the default neuromuscular controller during perturbed gait cycle differed little from the joint moments during unperturbed gait cycle (Fig. 4.). In contrast, the joint moments delivered by the neuromuscular controller with COM velocity feedback were higher in response to pelvis pushes and lower in response to pelvis pulls than during unperturbed gait cycles (Fig. 4.). This modulation of the ankle moment with perturbation direction is similar to the human behavior observed in the experimental dataset without exoskeleton [28]. This suggests that the subjects synergistically interact with the exoskeletons as altered responses of the subjects when walking with the exoskeletons would also alter the controller behavior, which is based on the subjects’ kinematics.
Exoskeleton assistance in perturbed walking. A The exoskeleton moment increased in response to pelvis push perturbations in the neuromuscular controller with COM feedback compared to the default neuromuscular controller and compared to steady-state walking assistance. B The type of controller had a significant influence on the average exoskeleton moment during the perturbed right stance phase after push perturbations [F(2,22) = 29.5001, p < 0.001]. C The assistive ankle moment decreased in response to pelvis pull perturbations in the neuromuscular controller with COM feedback compared to the default neuromuscular controller and compared to steady-state walking. D The type of controller had a significant influence on the average exoskeleton moment during the perturbed right stance phase after push perturbations [F(2,20) = 81.1381, p < 0.001]. A and C contain data of one representative subject. The bar plots in B and D contain data of all subjects with the dots representing the response of individual subjects and the bars the averages across subjects. A repeated measures anova with Tukey’s HSD post-hoc test was used to compare the exoskeleton moment between controllers
Human response to perturbation with exoskeleton assistance
The neuromuscular controller with COM feedback decreased COM displacement and muscle activity during balance recovery after perturbations compared to the default neuromuscular controller and the minimal impedance controller (Figs. 5, 6).
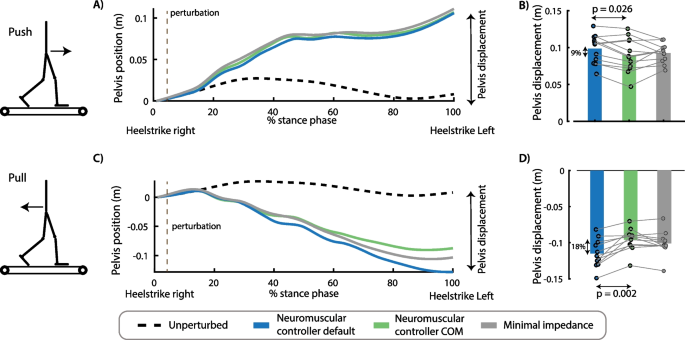
Effect of perturbation force and exoskeleton controller on pelvis displacement. The controller type influenced the movement of the pelvis after push perturbations [F(2,22) = 4.1593, p = 0.04)]. Forward displacement of the pelvis during the perturbed stance phase was smaller in the controller with COM feedback compared to the controller without COM feedback (p = 0.026) (A, B). The controller type also influenced the backward pelvis displacement after pull perturbations [F(2,20) = 21.92, p < 0.001]. Backward pelvis displacement at the first heel strike after the perturbation was smaller in the controllers with COM feedback compared to the controller without COM feedback (p = 0.002) (C, D). A and C contain data of one representative subject. The bar plots in B and D contain data of all subjects with the dots representing the response of individual subjects and the bars the averages across subjects. A repeated measures anova with Tukey’s HSD post-hoc test was used to compare the pelvis displacement between controllers
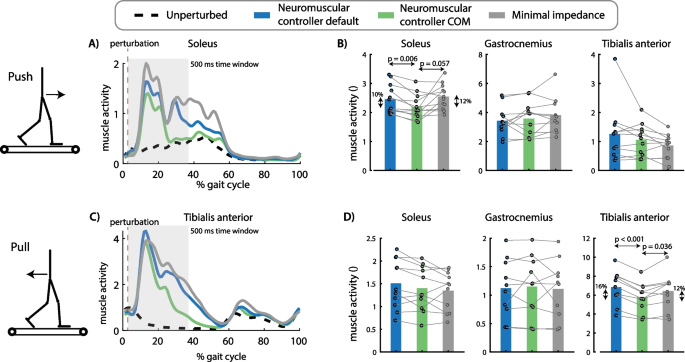
Effect of perturbation force and exoskeleton controller on muscle activity. The exoskeleton controller type influenced the increase in soleus activity after push perturbations [F(2,22) = 5.2763, p = 0.013]. Soleus activity in the first 500 ms after perturbation onset was smaller for the neuromuscular controller with COM feedback compared to the default neuromuscular controller (p = 0.006) and compared to the minimal impedance controller (although not significant, p = 0.057) (A, B). The exoskeleton controller type also influenced the increase in tibialis anterior activity after pull perturbations [F(2,18) = 15.1174, p = 0.0001]. Tibialis anterior activity was smaller in the neuromuscular controller with COM feedback compared to the default neuromuscular controller (p < 0.001) and compared to the minimal impedance controller (p = 0.036) (C, D). A and C contain data of one representative subject. The bar plots in B and D contain data of all subjects with the dots representing the response of individual subjects and the bars the averages across subjects. A repeated measures anova with Tukey’s HSD post-hoc test was used to compare the pelvis displacement between controllers
Push perturbations caused a forward movement of the subjects’ COM with respect to the treadmill (Fig. 5A, B) and an increase in soleus activity of the stance leg (Fig. 6A, B). The neuromuscular controller with COM feedback reduced the forward COM displacement during the stance phase with 9% compared to the default neuromuscular controller (p = 0.002) and resulted in similar COM displacements than the minimal impedance controller (p = 0.828) (Fig. 5A, B). The neuromuscular controller with COM feedback reduced stance leg soleus activity during the first 500 ms after the push perturbation with 10% compared to the default neuromuscular controller (p = 0.006) and with 12% compared to the minimal impedance controller (although not significant, p = 0.057) (Fig. 6A, B).
Pull perturbations caused a backward movement of the subjects’ COM with respect to the treadmill (Fig. 5C, D) and an increase in stance leg tibialis anterior activity (Fig. 6C, D). The neuromuscular controller with COM feedback reduced the backward COM displacement during the stance phase by 18% compared to the default neuromuscular controller (p = 0.002) and resulted in similar COM displacements than the minimal impedance controller (p = 0.201) (Fig. 5C, D). The neuromuscular controller with COM feedback reduced stance leg tibialis anterior activity during the first 500 ms after the pull perturbation with 16% compared to the default neuromuscular controller (p < 0.001) and with 12% compared to the minimal impedance controller (p = 0.036) (Fig. 6C, D).
Discussion
Exoskeletons can improve the efficiency of human walking [1,2,3,4,5, 30] but have currently limited ability to support balance control. We developed a balance supporting controller for an ankle exoskeleton that mimics the human ankle function during steady-state and perturbed walking. The controller reduced muscle activity in steady-state walking and during balance recovery after perturbations. The proposed biomimetic control strategy based on a neuromuscular model that includes muscle dynamics, local reflexes and supra-spinal balance pathways thus provides complete locomotion support including both effort and stability. This is especially important for the application of wearable robotic devices in aging and pathological populations with balance impairments.
Our control approach mimics intrinsic muscle dynamics and supra-spinal feedback control, which might both be important for its success in reducing muscle activity during balance recovery. Joint mechanical impedance is modulated during the stance phase of walking [31] and has important contributions to stabilize human walking [32]. Joint impedance originates both from muscle mechanical properties and muscle control. In our model, mechanical impedance originates from the force–length-velocity relationship in the virtual Hill-type muscle [33]. Mechanical impedance has been shown to be important to reduce the need for active control through delayed feedback [32, 34, 35]. However, mechanical impedance and local reflexes alone cannot explain observed changes in ankle torque after perturbations (Fig. 1, default neuromuscular model). The comparison of the controller with and without COM velocity feedback demonstrates that supra-spinal feedback is important for supporting balance control during walking with wearable robotic devices. The importance of task-level feedback is in line with previous research on the use of an ankle exoskeleton to support perturbed standing balance, where reductions in COM movement and muscle activity were larger with a controller that used feedback from body sway and sway velocity than local feedback from ankle angle and angular velocity [36]. Although we only tested our controller with perturbations applied to the pelvis and thus close to the COM, we have previously shown that ankle torques can also be described by delayed feedback from COM kinematics when walking is perturbed by sudden changes in treadmill belt speed [22, 23]. We chose to also mimic human feedback delays. This might be surprising given that controller performance in general decreases with increasing delays but was in line with our aim to closely match human balance control. Since we performed our experiments, a study was published that showed that reactive standing balance control can be augmented when ignoring this neural delay [37]. This study therefore indicates that we might be able to further reduce muscle activity and COM displacement by reducing COM feedback delays.
Our results suggest that human sensorimotor processing was unaltered by exoskeleton support. Similar as in perturbed walking without an exoskeleton [22], we found a correlation between the COM displacement and the change in ankle moment. Specifically, variability in the muscle response to perturbations can be explained by variability in COM movement (Additional file 1: Fig. S9–S10). We therefore infer that the reduction in COM excursion and velocity with the novel exoskeleton controller induced reductions in muscle activity in the absence of large changes in human balance control. An alternative way to evaluate how well balance is supported is by evaluating whether the exoskeleton compensates for the work done by the pusher as restoring balance requires generation or dissipation of mechanical energy to compensate for the perturbation and to return to the original steady-state gait cycle [38]. We found that the neuromuscular controller with COM feedback partially compensates for the energy dissipated by the pull perturbations in the stance phase during which the perturbation is given (Additional file 1: Figure S11). We performed additional exploratory analyses to evaluate whether the provided balance assistance elicited compensations in the contralateral leg or stepping. The neuromechanical controller with COM feedback reduced soleus activity in the contralateral leg with respect to the minimal impedance controller during both push and pull perturbations (Additional file 1: Fig S13). However, it might have slightly increased tibialis anterior activity in the contralateral leg with respect to the minimal impedance controller (Additional file 1: Fig S13). For all controllers, subjects mainly restored balance with an ankle strategy and not by adjusting foot placement (Additional file 1: Fig S12 and S14). These exploratory analyses further support our finding that the neuromuscular controller supports the ankle strategy to control balance after both push and pull perturbations.
It remains to be tested whether the proposed controller can support walking balance in patients with altered sensorimotor transformation underlying balance control. It has been documented that sensorimotor processing underlying standing balance control is altered in older adults and patients with Parkinson's disease [39]. In contrast to healthy adults, they also recruit antagonistic muscles in response to center of mass perturbations during standing [39]. It is largely unknown how sensorimotor processing underlying walking balance control is affected in persons with neurological disorders. It is therefore unclear whether patients with balance control deficits can also exploit the provided balance support. Whereas it seems unlikely that adapting the controller to mimic the balance control strategy of mobility impaired individuals would improve balance, it is unclear whether the control strategy inspired by healthy adults should be adapted for mobility impaired individuals.
We expect that the proposed balance controller can be extended to support balance across walking conditions given that the underlying COM feedback strategy explains human balance control across conditions. We previously demonstrated that COM feedback can explain corrective ankle moments across perturbation types (pelvis perturbations and support-surface translations) and gait speeds [22]. However, COM velocity feedback gains change with gait speed and throughout the stance phase. Faster walking is mechanically more stable and relies more on adjustment of foot placement to control balance due to the higher step frequency [25]. Hence, balance control during faster walking relies less on the modulation in the ankle moment. Implementing a controller with feedback gains that are modulated with gait speed and gait phase is feasible given that both can be estimated from wearable sensors (e.g. inertial measurement unit [40]. Adaptability of the balance correcting feedback pathways would complement the adaptability of the local feedback pathways. It has been shown that the default controller is able to reproduce steady state walking at various speeds in simulations [18] and results in speed-adaptive behavior when used to control a transtibial prosthesis [41].
Reductions in muscle activity do not imply reductions in metabolic energy consumption. It is hard to relate the observed 19% reduction in soleus activity during steady-state walking to a reduction in metabolic power. Metabolic power does not only depend on muscle activity but also on the operating conditions of the muscle. Previous studies have demonstrated that an external force provided by the exoskeleton in parallel with the compliant muscle–tendon unit can alter the operating length and velocity of the muscle and therefore undermine the energy efficiency [42, 43]. We expect that our controller can be further optimized to reduce metabolic cost. Previous research has demonstrated that the timing of assistance is important to reduce the metabolic cost [3]. Exoskeletons that were successful in reducing metabolic cost mainly provided assistance during push-off, when the muscle fibers are performing metabolically costly concentric work. Hence, only providing assistance when muscle fibers are shortening, and not simply based on muscle force as in the current implemented of our controller, might be beneficial to reduce metabolic cost. However, simultaneously reducing metabolic power and improving stability might require dedicated strategies.
Our observation that muscle activity is reduced with the default neuromuscular controller is in contrast with previous work. Shafer et al. found an increase in soleus activity during early stance and swing and an increase in metabolic power with a similar controller [17]. Multiple differences between study protocols may explain the discrepancy between both studies. First, Shafer et al. tested the controller at a walking speed of 1.25 m/s in [17] whereas our participants walked at 0.6 m/s. Second, we slightly modified the local feedback controller. We implemented a gradual change in feedback gains between stance and swing phases and identified control parameters based on experimental data whereas Shafer et al. [17] only did a sweep of two control parameters. A more gradual change in control parameters allowed us to better capture the biological torque. Without these adaptations, we overestimated the ankle torque in early stance, which might explain the increase in soleus activity during early stance in the experiments of Shafer et al. [38] (Additional file 1: Fig. S3).
Implementing the proposed controller in daily life requires wearable alternatives for the lab-based sensors, but we believe that these sensor are readily available. Laboratory-based sensors to measure the ground reaction force and COM velocity were used as input in the controller. We believe that these sensors can be easily replaced by foot switches to detect contact with the ground and an inertial measurement unit to estimate COM velocity [44]. Implementing the proposed controller in daily life situations also requires further validation in overground walking. The constant treadmill speed in our experiment and the requirement to stay on the treadmill might have shaped the balance recovery action of the subject and the interaction with the exoskeleton. Finally, we tested our controller on a wearable exoskeleton that was originally designed to support individuals with complete spinal cord injuries [45]. As a result, the exoskeleton was over-dimensioned for our study, explaining the relatively high weight (5 kg on each ankle–foot). For this reason, we did compare our controller’s performance to a minimal impedance controller rather than to walking without an exoskeleton. However, our controller is not device-specific and therefore applicable to other hardware designs such as transtibial prostheses. Given sufficiently light hardware, we expect our controller to reduce muscle activity with respect to walking without an exoskeleton but this remains to be demonstrated. Our current results can thus best be seen as a proof of principle for a biomimetic control design for balance support.
More at link.
No comments:
Post a Comment